Home | About us | Delivery | Advertisers | Login | Registration
Delivery on Sundays and holidays does not work!
- Medicines
- dietary supplementsVitamins
- Categories from A to Z
- Brands from A to Z
- Products from A to Z
- Medical equipment
- beauty
- Child
- Care
- Honey products appointments
- Herbs and herbal teas
- Medical nutrition
- Journey
- Making medicinesStock
Pharmacy online is the best pharmacy in Almaty, delivering medicines to Almaty. An online pharmacy or online pharmacy provides the following types of services: delivery of medicines, medicines to your home. Online pharmacy Almaty or online pharmacy Almaty delivers medicines to your home, as well as home delivery of medicines in Almaty.
my basket
Apteka84.kz is an online pharmacy that offers its customers medicines, medicinal and decorative cosmetics, dietary supplements, vitamins, baby food, intimate products for adults, medical equipment and thousands of other medical and cosmetic products at low prices. All data presented on the Apteka84.kz website is for informational purposes only and is not a substitute for professional medical care. Apteka84.kz strongly recommends that you carefully read the instructions for use contained in each package of medicines and other products. If you currently have any symptoms of the disease, you should seek help from a doctor. You should always tell your doctor or pharmacist about all the medicines you take. If you feel you need further help, please consult your local pharmacist or contact our GP online or by telephone.
© 2021 Pharmacy 84.
Korglykard (Korglikon) solution IV 0.6 mg cor 1 ml x10
MINISTRY OF HEALTH OF THE RUSSIAN FEDERATION
INSTRUCTIONS
on medical use of a medicinal product
CORGLICARD
Registration number: P N016062/01
Trade name of the drug: Corglikard
International nonproprietary name, lily of the valley leaf glycoside.
Dosage form: solution for intravenous administration.
Composition: 1 ml of solution contains the active ingredient corglycone - 0.60 mg,
excipients: chlorobutanol hemihydrate, water for injection.
Description: transparent liquid of slightly yellowish color.
Pharmacotherapeutic group: cardiotonic agent - cardiac glycoside.
KODATHS01AH.
Pharmacological properties
Pharmacodynamics
Purified preparation from the leaves of lily of the valley and its varieties. Cardiac glycoside has a positive inotropic effect. This is due to the direct inhibitory effect of sodium-potassium ATPase on the membranes of cardiomyocytes, which leads to an increase in the intracellular content of sodium ions and, accordingly, a decrease in potassium ions. An increased content of sodium ions causes activation of sodium/calcium metabolism, an increase in the content of calcium ions, as a result of which the rate of myocardial contraction increases.
As a result of an increase in myocardial contractility, the stroke volume of blood increases, the end-systolic and end-diastolic volumes of the heart decrease, which, along with an increase in myocardial tone, leads to a reduction in its size and, thus, to a decrease in the myocardial oxygen demand.
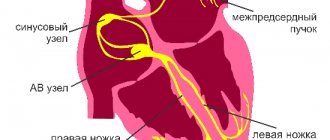
It has a negative chronotropic effect, reduces excessive sympathetic activity by increasing the sensitivity of cardiopulmonary baroreceptors. Due to the increase in the activity of the vagus nerve, it has an antiarrhythmic effect due to a decrease in the speed of impulses through the atrioventricular node and a lengthening of the effective refractory period. This effect is enhanced by a direct effect on the atrioventricular node and a sympatholytic effect.
The negative dromotropic effect manifests itself in increased refractoriness of the atrioventricular node.
With atrial fibrillation, cardiac glycosides help slow ventricular contractions, lengthen diastole, and improve intracardiac and systemic hemodynamics. Positive bathmotropic effect is manifested in subtoxic and toxic doses.
It has a direct vasoconstrictor effect, which is most clearly manifested in the absence of congestive peripheral edema. At the same time, the indirect vasodilating effect (in response to an increase in minute blood volume and a decrease in excessive sympathetic stimulation of vascular tone), as a rule, prevails over the direct vasoconstrictor effect, resulting in a decrease in overall peripheral vascular resistance.
When administered intravenously, the effect begins after 3-5 minutes and reaches a maximum after 25-30 minutes.
Pharmacokinetics
Distribution: binding to blood plasma proteins is negligible.
Excretion: practically does not undergo biotransformation in the liver and is excreted unchanged in the urine.
Indications for use
As part of the complex therapy of chronic heart failure of functional class II (in the presence of clinical manifestations) and functional class III-IV according to the NYHA classification, tachysystolic form of atrial fibrillation and atrial flutter of a paroxysmal and chronic course (especially in combination with chronic heart failure).
Contraindications
Glycoside intoxication, hypersensitivity to the drug, second degree atrioventricular block, Wolff-Parkinson-White syndrome, intermittent complete blockade, pregnancy and lactation.
Directions for use and doses
Administer intravenously slowly over 5-6 minutes (in 10-20 ml of 20% or 40% dextrose solution) 1-2 times a day. Adults are administered in a single dose of 0.5-1 ml, children aged 2 to 5 years - 0.2-0.5 ml, from 6 to 12 years - 0.5-0.75 ml. When administered 2 times a day, the interval between injections is 8-10 hours.
Higher doses for adults into a vein: single - 1.0 ml, daily - 2.0 ml.
Carefully
Atrioventricular block of the first degree, sick sinus syndrome without a pacemaker, the possibility of unstable conduction through the atrioventricular node, a history of Morgagni-Adams-Stokes attacks, hypertrophic subaortic stenosis, isolated mitral stenosis with a rare heart rate, cardiac asthma in patients with mitral stenosis (in the absence of the tachysystolic form of atrial fibrillation), acute myocardial infarction, unstable angina, arteriovenous shunt, hypoxia, heart failure with impaired diastolic function (restrictive cardiomyopathy, cardiac amyloidosis, constrictive pericarditis, cardiac tamponade), extrasystole, severe dilatation of the heart cavities, “ pulmonary" heart, electrolyte disorders: hypokalemia, hypomagnesemia, hypercalcemia, hyponatremia, hypothyroidism, alkalosis, myocarditis, old age, renal and hepatic failure, obesity.
Overdose
From the cardiovascular system: ventricular paroxysmal tachycardia, ventricular extrasystole (often bigeminy, polytopic ventricular extrasystole), nodal tachycardia, atrial fibrillation and flutter, atrioventricular block.
From the digestive tract: anorexia, vomiting, diarrhea, abdominal pain, intestinal necrosis.
From the nervous system and sensory organs: neuritis, manic-depressive syndrome, coloring of visible objects in a yellow-green color, flashing “flies” before the eyes, decreased visual acuity, perception of objects in a reduced or enlarged form.
Treatment: withdrawal of cardiac glycosides, administration of antidotes (unithiol, ethylenediaminetetraacetic acid), symptomatic therapy. Class I drugs (lidocaine, phenytoin) are used as antiarrhythmic drugs. For hypokalemia - intravenous administration of potassium chloride (6-8 g/day at the rate of 1-1.5 g per 0.5 liter of isotonic dextrose solution and 6-8 units of insulin, administered by drip over 3 hours). For severe bradycardia, atrioventricular block - M-anticholinergic blockers. It is dangerous to administer beta-agonists due to the possible potentiation of the arrhythmogenic effect of cardiac glycosides. In case of complete transverse block with Morgagni-Adams-Stokes attacks - temporary cardiac pacing.
Side effect
Side effects of Corglikard are associated with increased sensitivity of the patient to cardiac glycosides or overdose. From the cardiovascular system: arrhythmia, atrioventricular block.
From the central nervous system and sensory organs: drowsiness, confusion, sleep disturbances, headache, dizziness, delirious psychosis, decreased visual acuity.
From the hematopoietic organs: thrombocytopenia, thrombocytopenic purpura, nosebleeds.
From the digestive system: anorexia.
Other: allergic reactions.
Interaction with other drugs
Adrenergic agonists. The combined use of ephedrine hydrochloride, epinephrine hydrochloride or norepinephrine hydrotartrate, as well as selective beta-agonists with cardiac glycosides may contribute to the occurrence of cardiac arrhythmia.
Aminazine and other phenothiazine derivatives. The effect of cardiac glycosides decreases.
Anticholinesterase drugs. With simultaneous use of anticholinesterase drugs with cardiac glycosides, bradycardia increases. If necessary, it can be eliminated or weakened by the introduction of atropine sulfate.
Glucocorticosteroids. If hypokalemia occurs as a result of prolonged treatment with glucocorticosteroids, the undesirable effects of cardiac glycosides may increase.
Diuretics. When diuretics (which cause hypokalemia and hypomagnesemia, but increase the concentration of calcium ions in the blood) are combined with cardiac glycosides, the effect of the latter is enhanced. When using them simultaneously, you must adhere to the optimal dosage. You can periodically prescribe potassium-sparing diuretics (spironolactone, triamterene), which eliminate hypokalemia. However, hyponatremia may develop.
Potassium preparations. Under the influence of potassium preparations, the undesirable effects of cardiac glycosides are reduced.
Calcium preparations. When treating with cardiac glycosides, parenteral use of calcium preparations is dangerous, since the cardiotoxic effects (cardiac arrhythmias, etc.) are enhanced.
Ethylenediaminetetraacetic acid disodium salt. There is a decrease in the effectiveness and toxicity of cardiac glycosides.
Corticotropin preparations. The effect of cardiac glycosides under the influence of corticotropin may be enhanced.
Xanthine derivatives. Caffeine or theophylline drugs sometimes contribute to cardiac arrhythmias.
Sodium adenosine triphosphate. Sodium adenosine triphosphate should not be prescribed simultaneously with cardiac glycosides.
Ergocalciferol. With hypervitaminosis caused by ergocalciferol, the effect of cardiac glycosides may be enhanced due to the development of hypercalcemia. , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , ,
Narcotic analgesics. The combination of fentanyl and cardiac glycosides may cause hypotension.
Naproxen. In healthy people, the combined use of cardiac glycosides with naproxen does not affect the results of psychological testing.
Paracetamol. The clinical significance of this interaction has not been sufficiently studied, but there is evidence of a decrease in the excretion of cardiac glycosides by the kidneys under the influence of paracetamol.
special instructions
The likelihood of intoxication increases with hypokalemia, hypomagnesemia, hypercalcemia, hypernatremia, hypothyroidism, severe dilatation of the heart cavities, cor pulmonale, myocarditis, obesity, and old age. With severe mitral stenosis and normo- or bradycardia, heart failure develops due to a decrease in diastolic filling of the left ventricle. Strophanthin, increasing the contractility of the right ventricular myocardium, causes a further increase in pressure in the pulmonary artery system, which can provoke pulmonary edema or aggravate left ventricular failure. For patients with mitral stenosis, cardiac glycosides are prescribed when right ventricular failure occurs or in the presence of atrial fibrillation. Korglykon in Wolff-Parkinson-White syndrome, reducing atrioventricular conduction, promotes the conduction of impulses through accessory pathways - bypassing the atrioventricular node, provoking the development of paroxysmal tachycardia. As one of the methods for monitoring the level of digitalization when prescribing cardiac glycosides, monitoring their plasma concentration is used.
Release form.
Solution for intravenous administration 0.6 mg/ml.
During production at Opytny LLC, Ukraine.
1 ml in ampoules.
10 ampoules each along with instructions for medical use and an ampoule scarifier or ceramic cutting disc in a cardboard pack with a cardboard divider.
If there is a break ring or break point on the ampoule, do not put an ampoule scarifier or a ceramic cutting disc into the pack.
During production at Pharmaceutical LLC, Ukraine.
1 ml in ampoules.
10 ampoules each along with instructions for medical use and an ampoule scarifier or ceramic cutting disc in a cardboard pack with a cardboard divider.
5 ampoules are placed in a blister pack made of polyvinyl chloride film or polyvinyl chloride film and aluminum foil; 2 ampoules each, along with instructions for medical use and a scarifier or ceramic cutting disc, are placed in a cardboard pack.
10 ampoules are placed in a blister pack made of polyvinyl chloride film or polyvinyl chloride film and aluminum foil, 1 blister pack, along with instructions for medical use and a scarifier or ceramic cutting disc, is placed in a cardboard pack.
If there is a fracture ring or a point and a notch on the ampoule, an ampoule scarifier or a ceramic cutting disk is not placed in the pack.
Best before date
3 years. Do not use after the expiration date stated on the package.
Storage conditions
Store in original packaging at a temperature not exceeding 25°C.
Keep out of the reach of children
Conditions for dispensing from pharmacies
On prescription.
Registration Certificate Holder
LLC "Experienced", Ukraine, 61057, Kharkov region, Kharkov city, Vorobyova street, building 8.
, Ukraine, 61057, Kharkov region, Kharkov city, Vorobyova street, building 8.
Pharmaceutical LLC, Ukraine, 61013, Kharkov region, Kharkov city, Shevchenko street, building 22. Organization accepting consumer complaints:
Ukraine, 61057, Kharkov region, Kharkov city, Vorobyova street, building 8.
Tel/fax: +38
Director of LLC “Experimental plant “GNTsLS” , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , V.B. Demekhin