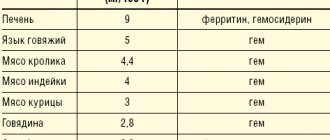
According to experts from the World Health Organization, about 1.6 billion people worldwide suffer from anemia, or 24.8% of the total population [1]. A significant proportion of cases of anemia are associated with iron deficiency [2], which may be a consequence of insufficient dietary intake, in particular in chronic alcoholism, increased need (childhood and adolescence, pregnancy, postpartum period), chronic blood loss, malabsorption (for example, in inflammatory bowel diseases) [3]. In case of functional iron deficiency, for example, during treatment with erythropoiesis stimulants, normal or even increased total iron content in the body turns out to be insufficient against the background of an increased need for bone marrow iron [4]. Hepcidin, which is synthesized in the liver and suppresses the absorption of iron in the intestine and its release from the depot and macrophages, takes part in the regulation of iron metabolism [5]. An increase in hepcidin concentration contributes to the development of anemia in chronic diseases, nephrogenic anemia with possible resistance to erythropoiesis stimulants [6].
Anemia occurs in a third of women of reproductive age, and during pregnancy its frequency reaches 23–52% [2]. In women, iron deficiency anemia (IDA) most often develops with heavy and prolonged menstruation (more than 5 days), dysfunctional uterine bleeding, and uterine fibroids [7]. With low iron intake, anemia can develop even with relatively little blood loss [8]. The high incidence of anemia during pregnancy reflects a significant increase in iron requirements due to increased erythropoiesis in the woman and fetal growth [9].
In case of IDA, an increase in iron intake from food does not compensate for its deficiency, because the maximum absorption of iron is only 2.5–3 mg/day [9]. In this regard, the main method of treating IDA is oral or intravenous administration of iron supplements.
Intravenous iron preparations and indications for their use
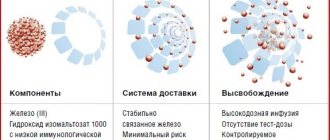
For intravenous administration, iron-carbohydrate compounds (carboxymaltose, saccharate, gluconate and iron dextran) are used. The carbohydrate shell stabilizes the complex and slows down the release of iron in the blood. Low molecular weight complexes, such as ferric gluconate, are less stable and more rapidly release iron into the plasma, which, in its free form, can catalyze the formation of reactive oxygen species that cause lipid peroxidation and tissue damage [26]. A significant portion of the dose of such drugs is excreted through the kidneys in the first 4 hours after taking the drug and is not used for erythropoiesis. The disadvantage of iron dextran, which has high molecular weight and stability, is the increased risk of allergic reactions [26]. Ferric carboxymaltose (Ferinject) is a stable high molecular weight complex that provides a slow and physiological release of iron. It has less immunogenic potential and, unlike iron saccharate and gluconate, can be administered in a high dose [10].
In a randomized controlled trial, ferric carboxymaltose was compared with ferric dextran in 160 patients with IDA [11]. The effectiveness of the two drugs did not differ significantly, however, hypersensitivity reactions were not recorded in any of the patients in the ferric carboxymaltose group and were observed among 10.3% of patients in the comparison group (p = 0.003). The incidence of skin reactions was also significantly lower when treated with iron carboxymaltose (7.3 and 24.4%, respectively; p = 0.004).
When using iron carboxymaltose, 1000 mg of iron can be administered intravenously over 15 minutes, while the maximum dose of iron in the form of sucrose is 500 mg and is administered over 3.5 hours, and the duration of infusion of iron dextran reaches 6 hours, according to the instructions for medical use. Moreover, in the last two cases, before starting the infusion, it is necessary to administer a test dose. The maximum recommended single doses of most iron preparations for intravenous administration are lower than those of ferrous carboxymaltose, so repeated administration is necessary to replenish iron stores, while when using ferrous carboxymaltose, the frequency of infusions is correspondingly smaller.
For mild anemia, iron supplements are most often prescribed orally. The main advantage of oral iron supplements over intravenous ones is ease of use, although this is offset by the high frequency (10–40%) of side effects from the gastrointestinal tract (GIT [10]) and the need for long-term treatment. Intravenous administration of iron supplements allows you to quickly replenish iron reserves in the body to restore hemoglobin (Hb) concentrations. This effect is of particular importance in more severe anemia, especially in patients with cardiovascular disease, as well as in preoperative anemia, when surgery cannot be delayed [12]. It should be taken into account that preoperative anemia (including mild) is not only common in clinical practice, but is also associated with a significant increase in the risk of death and other complications within 30 days after surgery [13]. According to a systematic review of 24 randomized and 15 non-randomized trials [14], intravenous iron supplementation for preoperative anemia provided a faster and more reliable effect than oral administration. Intravenous iron is used by patients receiving treatment with drugs that stimulate erythropoiesis, patients with chronic kidney disease, inflammatory bowel diseases, and malignant tumors [15]. The cause of iron deficiency in cancer patients may be not only bleeding or impaired iron supply during anorexia or after resection of gastrointestinal tumors, but also other factors, in particular an increase in the secretion of hepcidin, which suppresses the absorption of iron in the intestine and its release from depots and macrophages [5, 15]. In several randomized clinical trials, intravenous iron supplementation increased the response rate to epoetin treatment in cancer patients from 25–70 to 68–93% [16], while oral medications were ineffective or ineffective in such patients. The transition to intravenous administration of iron supplements is also indicated for patients for whom treatment with oral medications is ineffective or poorly tolerated [17].
Today, the world medical community is also discussing the possibility of using iron supplements for latent deficiency, which is not accompanied by anemia, but can cause fatigue. The multicenter, randomized, placebo-controlled PREFER trial included 290 women with fatigue (Piper Fatigue Scale [PFS] score ≥5), iron deficiency (ferritin <50 μg/L and transferrin saturation <20% or ferritin <15 μg /l) and normal or borderline Hb level (≥11.5 g/dl), who were given a single dose of ferric carboxymaltose (1000 mg iron) or saline [18]. Fatigue decreased in 65.3% of women receiving ferric carboxymaltose and in 52.7% of patients in the placebo group (p = 0.03), and the PFS index decreased by at least half in 33.3 and 16.4% of women respectively (p<0.001). Administration of ferric carboxymaltose resulted in greater improvements in mental state indices and cognitive function. In conclusion, the results of this study showed that intravenous iron supplementation in women with iron deficiency can lead to a decrease in fatigue and improvements in quality of life and cognitive function, even in the absence of anemia. In Russia today, iron carboxymaltose is used, according to the approved instructions, only for iron deficiency anemia.
Choosing the dose of intravenous iron supplements
Before starting treatment with intravenous iron, the optimal cumulative dose of the drug should be determined, which should not be exceeded. The cumulative dose required to restore Hb levels in the blood and replenish iron reserves in the body is traditionally calculated using the Ganzoni formula: cumulative iron deficiency (mg) = body weight [kg] × (15 actual Hb) [g/dl] × 2, 4+500 [mg] [19]. To convert the Hb level to mmol/l, the value in g/dl should be multiplied by 1.61145 [10]. The instructions for use of some drugs, for example Ferinject, Venofer and Cosmofer, contain tables that allow you to calculate the required dose taking into account the actual Hb content and the patient’s body weight. In practice, the total dose of iron administered intravenously is usually about 1000 mg [19]. In fact, some patients with IDA may require a higher total dose for the entire course.
The severity of iron deficiency in patients with IDA who were administered intravenous iron supplements in clinical trials, and the potential benefit of increasing their total dose, were studied in the work of T. Koch et al. [19]. Next, we review the results of 7 studies that included patients with IDA of various origins, including women with postpartum anemia or anemia that developed after severe uterine bleeding, patients with gastrointestinal diseases and nephrogenic anemia [11, 20–25]. All studies used ferric carboxymaltose and compared it with oral or other intravenous iron supplements. Two studies specifically examined the efficacy and safety of ferric carboxymaltose at a higher total dose of 1500 mg. In these studies, patients in the comparison groups received standard therapy (oral or intravenous iron supplements at the investigator's choice) [24] or intravenous iron sucrose at a dose of 1000 mg [25].
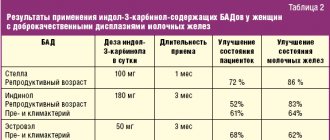
The degree of iron deficiency was calculated using the Ganzoni formula/modified Ganzoni formula. In patients with baseline transferrin saturation >20% and ferritin concentration >50 ng/ml, the 500 mg required to replenish iron stores was not taken into account when calculating iron deficiency [19]. The average cumulative doses of iron intake in 5 clinical studies of iron carboxymaltose intake are presented in Table. 1.
J. Onken et al. studied the efficacy and safety of two 750 mg infusions of ferric carboxymaltose at weekly intervals compared with oral iron supplementation in 1011 patients with IDA of various origins, who were divided into two cohorts [24]. The first cohort consisted of 507 patients for whom treatment with oral iron preparations for 14 days did not lead to a significant increase in Hb concentration (by up to 10 g/l). This cohort compared iron carboxmaltose given as a double infusion (Group A) with oral iron 325 mg/day for 14 days (Group B). After 35 days, the Hb concentration in the two groups increased by an average of 15.7 and 8.0 g/L, respectively (p=0.001), and the proportion of patients in whom it reached at least 120 g/L was 57.0 and 29.1%, respectively (p=0.001) (Fig. 1). The second cohort included 504 patients who were intolerant to oral iron supplements. This cohort received ferric carboxymaltose (group C) or other intravenous iron supplements (group D). The total iron dose in the two groups averaged 1432 and 813 mg, respectively. The average Hb concentration 35 days after the administration of iron carboxymaltose at a higher dose increased by 29.0 g/l, and with the introduction of other iron preparations - by 21.6 g/l (p = 0.001). The benefits of a higher dose of iron were also shown when analyzing the number of patients who achieved an Hb concentration of at least 120 g/l; their number was 50.6 and 24.5% in the two groups, respectively (p = 0.001). In summary, this study demonstrated that two 750 mg infusions of ferric carboxymaltose given one week apart are effective and safe treatments for IDA when oral iron supplements are ineffective or poorly tolerated.
The REPAIR-IDA study compared the efficacy and safety of intravenous ferric carboxymaltose (two 750 mg infusions at weekly intervals) and intravenous iron sucrose (up to 5 200 mg infusions over 14 days) in 2584 patients with IDA and pre-dialysis chronic kidney disease (CKD). ) [25]. The primary efficacy outcome was the mean change from baseline in Hb concentration at 56 days, and the composite primary safety endpoint was the incidence of death from any cause, non-fatal myocardial infarction and stroke, unstable angina, chronic heart failure, arrhythmias, episodes of hypertension and hypotension. The total dose of iron was 1464 mg in the ferric carboxymaltose group and 963 mg in the comparison group. The initial Hb concentration was comparable in the two groups (103.1 and 103.2 g/L, respectively).
The Hb content increased by an average of 11.3 g/l when iron carboxymaltose was administered at a dose of 750 mg twice at weekly intervals and by 9.2 g/l when iron sucrose was administered at a total dose of 5 injections - 1000 mg. The proportion of patients whose Hb concentration increased by more than 10 g/l after 56 days after administration of iron carboxymaltose was higher than after administration of iron sucrose (48.6 and 41.0%). Moreover, the proportion of patients who required repeated intravenous administration of iron after 56–90 days in the main group was significantly lower than in the comparison group (5.6 and 11.1%, respectively; p<0.001; Fig. 2) [19 ].
Significant benefits of intravenous administration of a higher dose of iron were also revealed when analyzing the number of patients whose Hb concentration increased to 110 and 120 g/l within 56 days or by at least 10 g/l (Table 2) [19] . The time to these changes in Hb concentration was shorter in the ferric carboxymaltose group (Fig. 3) [19]. The safety of the two intravenous iron regimens did not differ significantly.
Day 56 was the last day of the study. At the discretion of the investigator, repeated administration of the iron supplement was allowed from days 56 to 90 [19].
Conclusion
In clinical practice, physicians usually discuss the possibility of intravenous iron supplementation in patients with IDA when oral iron supplementation is ineffective or poorly tolerated, although in many situations intravenous iron administration is the method of choice. An example is severe or preoperative anemia, when it is necessary to achieve a progressive increase in Hb concentration in order to avoid adverse outcomes or not to delay surgery.
Oral iron supplements may not be effective enough in the treatment of IDA in patients with inflammatory bowel disease, cancer, and CKD. Analysis of the estimated iron deficiency in clinical studies of patients with IDA showed that on average it can be about 1500 mg. Thus, the total dose of iron, which often does not exceed 1000 mg, may be insufficient for some patients with IDA. The results of randomized clinical trials indicate that intravenous iron supplementation up to a cumulative dose of 1500 mg allows for a more rapid significant increase in Hb concentration and increases the time to repeated iron infusions compared with those with a dose of 1000 mg.
To administer high doses of iron, iron carboxymaltose can be used, a single dose of which can be 1000 mg. Accordingly, a larger dose can be administered by repeated infusions of the drug at one-week intervals, whereas when using iron sucrose, a similar dose will require a larger number of infusions.
Classification of drugs containing iron
Preparations containing iron are classified into:
- iron preparations for oral administration (oral): iron sulfate, iron fumarate, iron saccharate, iron hydroxypolymaltose complex;
- iron preparations for parenteral use (injection): iron hydroxypolymaltose complex, iron hydroxysucrose complex, iron carboxymaltose, iron dextran;
- combined preparations: iron hydroxypolymaltose complex + folic acid; iron sulfate + folic acid;
- iron ammonium citrate + folic acid + cyanocobalamin;
- iron sulfate + ascorbic acid;
- iron sulfate + D, L-serine;
- iron gluconate + manganese gluconate + copper gluconate;
Iron compounds are also included in multivitamin preparations containing mineral complexes.