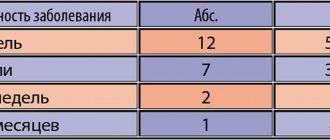
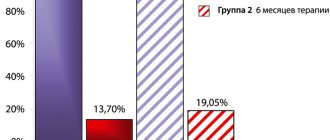
Pharmacodynamics and pharmacokinetics
Pharmacodynamics
A monophasic combination drug that suppresses the secretion of gonadotropic hormones, inhibits the maturation of follicles and suppresses ovulation. The hormonal substances included in the composition increase the viscosity of the mucus of the vagina and cervical canal and change the endometrium, which prevents the penetration of sperm into the cervical canal, on the one hand, and reduces the likelihood of egg implantation, on the other. Affect the movement of sperm in the fallopian tubes.
Pharmacokinetics
Gestodene is quickly absorbed after administration and after 1 hour its maximum content is noted in the blood. In the blood, it binds to albumin and special globulin (SHBG) by 69%. When taken daily, the content of gestodene in the blood increases 4 times. While taking the second half of the tablets, a state of equilibrium of its level in the blood is noted, the decrease of which occurs in two phases. Gestodene is completely metabolized and is excreted in the form of metabolites in urine and bile (half-life 24 hours).
Ethinyl estradiol is also completely absorbed from the intestine. Its content in serum peaks after 1-2 hours. The decrease in the level of the substance in the blood occurs in two phases. Ethinyl estradiol in the form of metabolites is excreted in bile and urine. Equilibrium concentration is achieved after 5-6 days from the start of taking the drug.
Vaginal ring
The hormonal vaginal ring (NovaRing) contains etonogestrel and ethinyl estradiol (daily release 15 mcg + 120 mcg, respectively). The ring is installed for three weeks, after which it is removed and a week-long break is maintained. On day 29 of the cycle, a new ring is inserted.
The dosage of ethinyl estradiol in the vaginal ring is lower than that of COCs, due to the fact that absorption occurs directly through the vaginal mucosa, bypassing the gastrointestinal tract. Due to the complete suppression of ovulation and regular release, independent of the patient, the effectiveness is higher than that of COCs (0.3–6%). Another advantage of the ring is the low likelihood of dyspeptic side effects. Some patients experience vaginal irritation and discharge. In addition, the ring may accidentally slip out.
The effect of hormonal contraceptives on libido has not been sufficiently studied; research data are contradictory and depend on the average age in the sample and gynecological diseases, the drugs used, and methods for assessing the quality of sexual life. In general, 10–20 percent of women may experience a decrease in libido while taking medications. In most patients, the use of GCs does not affect libido [10].
Acne and hirsutism usually have low levels of sex hormone binding globulin (SHBG). COCs increase the concentration of this globulin, having a beneficial effect on the condition of the skin.
Contraindications
- angina pectoris, hypertension, myocardial infarction;
- history of cerebrovascular accidents, including transient ones;
- presence of risk factors for thrombosis ;
- diabetes mellitus complicated by vascular damage;
- pancreatitis;
- severe liver and kidney diseases;
- decreased blood clotting;
- hormone-dependent breast tumors ;
- suspected pregnancy ;
- bleeding from the genital tract of unknown etiology;
- epilepsy;
- retinal vascular thrombosis;
- thrombosis of the veins of the extremities;
- overweight;
- hypersensitivity to gestodene or ethinyl estradiol.
Subtleties of application
Estrogen in COCs helps eliminate LDL and increase HDL and triglyceride levels. Progestins counteract estrogen-induced changes in lipid levels in the body.
- For acne, medications containing cyproterone acetate, drospirenone or desogestrel are prescribed as a progestin. COCs containing cyproterone acetate and ethinyl estradiol are more effective for acne than the combination of ethinyl estradiol and levonorgestrel [4].
- For hirsutism, medications containing progestogens with antiadrogenic properties are recommended: cyproterone acetate or drospirenone [5].
- Combinations of estradiol valerate and dienogest are more effective in reducing menstrual blood loss than ethinyl estradiol and levonorgestrel [6]. In addition, the intrauterine system is indicated for the treatment of menorrhagia.
- Preparations containing drospirenone 3 mg and ethinyl estradiol 20 mcg are recognized as the most effective combination for correcting PMS symptoms, including those of a psychogenic nature [7].
- Taking oral contraceptives increases systolic blood pressure (BP) by 8 mmHg. Art., and diastolic - by 6 mm Hg. Art. [8]. There is evidence of an increased risk of cardiovascular events in women taking COCs [9]. Due to the increased likelihood of developing myocardial infarction and stroke in patients with arterial hypertension, when prescribing COCs, the benefit/risk ratio must be carefully weighed.
- In non-smoking women under 35 years of age with compensated hypertension, COCs can be prescribed with careful monitoring of blood pressure during the first months of use.
- In cases of increased blood pressure while taking COCs or in women with severe hypertension, an intrauterine system or DMPA is indicated [10].
- The selection of a contraceptive for patients with dyslipidemia must be carried out taking into account the effect of the drugs on lipid levels (see Table 5).
- Because the absolute risk of cardiovascular events in women with controlled dyslipidemia is low, COCs containing 35 mcg or less of estrogen can be used in most cases. For patients with LDL levels above 4.14 mmol/l, alternative means of contraception are indicated [8].
- The use of COCs in women with diabetes mellitus accompanied by vascular complications is not recommended [2]. A suitable option for hormonal contraception for diabetes mellitus is the intrauterine levonorgestrel-releasing system, and, as a rule, no dose adjustment of hypoglycemic drugs is required.
- The results of epidemiological studies examining the risk of myocardial infarction when oral contraceptives are prescribed to women who smoke are contradictory. Due to limited convincing data, COCs are recommended to be prescribed with caution to all women over 35 years of age who smoke.
- Obesity with a body mass index of 30 kg/m2 and above reduces the effectiveness of COCs and transdermal GCs [8]. In addition, the use of COCs in obesity is a risk factor for venous thromboembolism. Therefore, the method of choice for such patients is mini-pills (gestagen-containing tablet contraceptives) and intrauterine contraceptives (levonorgesterel-releasing system).
- Use of COCs with estrogen dosages of less than 50 mcg in nonsmoking, healthy women over 35 years of age may have beneficial effects on bone density and vasomotor symptoms during perimenopause [8]. This benefit must be considered in light of the risk of venous thromboembolism and cardiovascular factors. Therefore, women of the late reproductive period are prescribed COCs individually.
List of sources
- Van Vliet HAAM et al. Biphasic versus triphasic oral contraceptives for contraception //The Cochrane Library. — 2006.
- Omnia M Samra-Latif. Contraception. Available from https://emedicine.medscape.com
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53,297 women with breast cancer and 100,239 women without breast cancer from 54 epidemiological studies. Lancet 1996; 347(9017):1713–1727.
- Carlborg L. Cyproterone acetate versus levonorgestrel combined with ethinyl estradiol in the treatment of acne. Results of a multicenter study. Acta Obstetricia et Gynecologica Scandinavica 1986;65:29–32.
- Batukan C et al. Comparison of two oral contraceptives containing either drospirenone or cyproterone acetate in the treatment of hirsutism. Gynecol Endocrinol 2007;23:38–44.
- Fruzzetti F, Tremollieres F, Bitzer J. An overview of the development of combined oral contraceptives containing estradiol: focus on estradiol valerate/dienogest. Gynecol Endocrinol 2012;28:400–8.
- Lopez LM, Kaptein AA, Helmerhorst FM. Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database Syst Rev 2012.
- Armstrong C, Coughlin L. ACOG releases guidelines on hormonal contraceptives in women with coexisting medical conditions. — 2007.
- Carr BR, Ory H. Estrogen and progestin components of oral contraceptives: relationship to vascular disease. Contraception 1997; 55:267–272.
- Burrows LJ, Basha M, Goldstein AT. The effects of hormonal contraceptives on female sexuality: a review. The journal of sexual medicine 2012; 9:2213–23.
Side effects of Logest
Low-dose hormonal contraceptives (containing minimal doses of estrogen and progesterone) are less likely to cause adverse reactions than high-dose ones, but you need to be aware of them:
- soreness and enlargement of the mammary glands, discharge from them;
- spotting and uterine bleeding;
- changes in vaginal secretion;
- depression , increased fatigue , headache (in the first months of use);
- abdominal pain, nausea and vomiting ;
- visual impairment;
- rash , erythema multiforme and nodosum , itching ;
- acne , baldness ;
- jaundice;
- swelling and weight gain;
- development of thrombosis and thromboembolism .
Since the use of hormonal contraceptives causes side effects, they are prescribed only by a doctor after a thorough examination and determination of the “benefit-risk” of their use. After discontinuation of the drug, the menstrual cycle is restored. Pregnancy after discontinuation of Logest occurs within a year.
Transdermal Therapeutic System (TTS)
The transdermal therapeutic system patch is applied for 7 days. The used patch is removed and immediately replaced with a new one on the same day of the week, on the 8th and 15th days of the menstrual cycle.
TTS appeared on the market in 2001 (“Evra”). Each patch contains a week's supply of norelgestromin and ethinyl estradiol. TTC is applied to dry, clean skin of the buttocks, abdomen, outer surface of the upper arm or torso with minimal hair growth. It is important to monitor the density of TTC attachment every day and not apply cosmetics nearby. Daily release of sex steroids (203 mcg norelgestromin + 33.9 mcg ethinyl estradiol) is comparable to that of low-dose COCs. On the 22nd day of the menstrual cycle, the TTS is removed and a new patch is applied 7 days later (on the 29th day).
The mechanism of action, effectiveness, disadvantages and advantages are the same as those of COCs.
Contraceptive pills Logest, instructions for use (Method and dosage)
The tablets are taken orally every day, in the order indicated on the package, preferably at the same time, without a break for 21 days. Then there is a break of 7 days, during which bleeding appears, after which they move on to taking new tablets.
If the woman has not previously taken hormonal contraceptives
Reception begins on the first day of the menstrual cycle. If you start taking it on the 2nd - 4th day of the cycle, then it is necessary to use barrier contraception for the first week of taking the pills.
Switching from other combined contraceptives
It is necessary to immediately start taking this contraceptive after taking the active tablet of the previous hormonal contraceptive.
When switching from intrauterine hormonal contraception containing progestogen, intradermal implants and injections, mini-pills
This drug is taken any day after finishing the mini-pill. When using injections or implants, the first tablet is taken on the day the intradermal implant is removed or the contraceptive injection ends. However, in all cases, barrier contraception is prescribed for a week.
How to take the drug after an early abortion
The drug is started immediately. In this case, there is no need for additional contraception.
Birth control pills after childbirth or late pregnancy abortion
Taken 21-28 days after physiological birth (or induced abortion performed in the second trimester). In case of a later start, a barrier method of contraception is used for a week.
If you miss taking a pill, there are separate instructions for using Logest.
Logest
Low-dose monophasic combined oral contraceptive based on estrogen (ethinyl estradiol) and progestogen (gestodene). The contraceptive effect is achieved by suppressing ovulation, changing the properties of cervical mucus and changing the endometrium. Sperm cannot penetrate the uterus, and the fertilized egg cannot attach to the surface of the endometrium.
In addition to protecting against unwanted pregnancy, Logest has a beneficial effect on the female body: the menstrual cycle becomes more regular, the pain and intensity of menstruation decreases, and the risk of iron deficiency anemia decreases.
Contraindications to taking Logest may include:
- thrombosis and thromboembolism;
- myocardial infarction, angina pectoris;
- migraine;
- diabetes mellitus with vascular complications;
- damage to the valvular apparatus of the heart, heart rhythm disturbances, diseases of the cerebral vessels or coronary arteries of the heart;
- high blood pressure;
- pancreatitis with severe hypertriglyceridemia currently or in history;
- liver failure and severe liver diseases, liver tumors;
- hormone-dependent malignant diseases (including genital organs or mammary glands) or suspicion of them;
- vaginal bleeding of unknown origin;
- prolonged immobility, serious injuries and surgical interventions.
Logest should not be taken during pregnancy and lactation. If pregnancy occurs while taking the drug, it should be stopped immediately. There is no need to fear for possible pathologies of the fetus, since extensive clinical studies prove that taking hormonal contraceptives in the early stages of pregnancy does not have a significant negative effect on the fetus.
With extreme caution and with constant monitoring, Logest can be prescribed in the following cases:
- obesity;
- thrombophlebitis;
- otosclerosis with hearing loss;
- idiopathic jaundice or itching during previous pregnancy;
- migraine;
- congenital hyperbilirubinemia (Gilbert, Dubin-Johnson and Rotor syndromes);
- diabetes;
- sickle cell anemia;
- arterial hypertension.
Directions for use and doses
Logest is taken as one tablet per day, preferably at the same time. The period of continuous use is 21 days, until all the tablets from the package are consumed. This is followed by a week-long break, during which menstrual-like bleeding usually occurs.
Start of reception
If you have not taken any hormonal contraceptives in the previous month, then the first Logest tablet is taken on the first day of the menstrual cycle, that is, on the first day of menstrual bleeding. It is possible to postpone the start of use to days 2-5 of the menstrual cycle, but in this case, barrier methods of contraception should be additionally used for a week.
If you are switching from another combined oral contraceptive, then the first Logest tablet is taken the next day after taking the last active tablet of the previous drug. It is possible to take the usual one-week break between drugs from different packages - if the package of the previous drug contains 21 tablets. If it contains 28 tablets, then the first Logest tablet is taken the next day after taking the last inactive tablet.
If you are making a transition from oral contraceptives containing only gestagens (mini-pills), then the first Logest tablet can be taken any day, without any break.
If you are making a transition from injection methods of contraception, then the first Logest tablet is taken on the day when the next injection would have been given.
If you are switching from an intrauterine contraceptive (for example, Mirena), then the first Logest tablet is taken on the day of its removal.
In all three cases of transition from gestagen-containing contraceptives (mini-pills, injections, intrauterine contraceptives) additional protective measures must be used within a week.
Starting after an abortion or childbirth
If the abortion occurred in the first trimester of pregnancy, then you can start taking Logest immediately. Additional contraceptive protection is not required.
If abortion or childbirth occurred in the second trimester of pregnancy, then taking Logest should be started 21-28 days after childbirth or abortion in the second trimester of pregnancy. If use is started later, it is necessary to use an additional barrier method of contraception during the first 7 days of taking the pill. However, if a woman has already been sexually active, pregnancy should be excluded before taking Logest or she must wait until her first menstruation.
If you forget to take your pill
If the delay is less than 12 hours, the contraceptive effect is not reduced. The drug still has its effects on the body. Therefore, in such a situation, you need to take the pill as soon as possible. No other measures are required. The subsequent tablet is taken according to the usual schedule.
If the delay is more than 12 hours, the contraceptive effect is reduced and conception becomes more likely. Actions in such a situation depend on which week of admission it occurred. You also need to always remember two rules:
- Reception of Logest should not be interrupted for a period exceeding seven days.
- In order for the maximum contraceptive effect to be achieved, it is necessary to take the drug for at least seven days.
Actions when the interval between two tablets exceeds 36 hours (delay in taking more than 12 hours):
First week of taking the drug
You need to take the missed pill as soon as possible - if the delay is approaching 24 hours, then you need to take two pills at the same time. Then the intake continues according to the usual schedule, but barrier methods of protection are also used during the week. It is necessary to take into account that if there was a sexual contract during the week before missing the pill, there is a possibility of pregnancy. Remember: the more pills you miss, and the closer they are to the week break, the greater the chance of pregnancy. In other words, a missed pill in the third week of use entails a greater likelihood of pregnancy than a missed pill in the first week.
Second week of taking the drug
Take the missed pill as soon as possible, then proceed according to your usual schedule. If a woman is confident that she adhered to her dosage schedule during the week before missing the pill, no additional precautions are required. If the previous appointment occurred with serious deviations from the schedule, then it makes sense to additionally use barrier methods.
Third week of taking the drug
If you miss taking a pill in the third week, the risk of a decrease in the contraceptive effect, as well as the risk of possible pregnancy, is inevitable. You can act in such a situation according to two schemes.
First scheme
- Take the missed pill as soon as possible, then take the drug according to your usual schedule.
- When all the pills from the current package are drunk, the next package is moved on - that is, without a seven-day break.
With this regimen, the onset of menstrual-like bleeding in the current cycle is unlikely; scanty spotting and breakthrough bleeding may occur while taking the second package.
Second scheme
- We consider the current packaging to be complete. We don’t take the remaining pills in it - we take a week’s break, the first day of which is considered the day you missed taking a pill.
- After the break, we begin taking tablets from the next package.
If bleeding occurs during the break, pregnancy must be ruled out.
Cases where vomiting or diarrhea occurs within 4 hours after taking a pill should be regarded as missing a pill. And act according to the recommendations presented above.
Possible side effects when taking Logest may be:
- soreness and tension of the mammary glands, enlargement of the mammary glands, discharge from the mammary glands;
- discharge and breakthrough uterine bleeding;
- headache, migraine;
- change in libido;
- emotional instability;
- poor tolerance to contact lenses, blurred vision;
- nausea, vomiting, abdominal pain;
- skin rash, itching, chloasma;
- erythema nodosum, erythema multiforme;
- cholestatic jaundice;
- swelling;
- change in body weight;
- allergic reactions;
- thrombosis, thromboembolism.
Please note: a number of medications may reduce the contraceptive effect of Logest. These are primarily: phenytoin, barbiturates, primidone, carbamazepine and rifampicin, oxcarbazepine, topiramate, felbamate, ritonavir, griseofulvin, ampicillin, tetracycline and preparations containing St. John's wort.
Please also pay attention to some points that are important to understand when taking Logest.
- In case of planned surgery, you need to stop taking the drug four weeks in advance, and it is recommended to resume taking it no earlier than two weeks after the end of the immobilization period.
- If you have been prescribed medications that affect microsomal enzymes, then during the course of treatment, as well as within 28 days after its completion, a decrease in the contraceptive effect of Logest is noted. In this regard, it is recommended to additionally use barrier methods of contraception. At the same time, if you have taken all the tablets from the package, and 28 days have not yet passed, you need to move on to the next package of Logest without a week's break.
- You should consult a doctor in cases where you experience:
- leg pain,
- severe chest pain
- sudden shortness of breath,
- causeless cough,
- any unusual, severe, long-lasting headache;
- problems with vision or speech;
- dizziness, loss of consciousness;
- weakness;
- "acute belly"
- Women suffering from diabetes, high blood pressure, and recurrent headaches should be especially carefully observed by a doctor.
- Preventive examinations by a gynecologist should be performed every six months.
- Logest is not recommended for smoking women over 35 years of age, as it may increase the risk of developing complications of the cardiovascular system.
Taking the drug should be stopped if, while taking:
- there is an increase in the frequency and intensity of headaches and migraines;
- epileptic seizures occur;
- signs of depression increase;
- surgical intervention is pending (appointment is stopped 4 weeks before the planned operation and can be resumed 1 week after the patient is mobilized).
Please note: intermenstrual bleeding may occur in the first three months of taking Logest. If they occur after regular cycles have formed, pregnancy and malignant neoplasms should be excluded.
If menstrual-like bleeding does not occur during the period without taking pills (the week between two packs) and the dosage regimen was violated in the previous cycle, it is necessary to take a pregnancy test.
Interaction
There is a decrease in contraceptive protection when taking ampicillins and tetracyclines , since they reduce the circulation of estrogen in the liver, lowering the concentration of ethinyl estradiol.
Sulfonamides, butadione, analgin, amidopyrine enhance the metabolism of steroid hormones included in Logest.
Medicines: barbiturates, carbamazepine, ritonavir, rifampicin, oxcarbazepine, griseofulvin reduce the contraceptive effectiveness of Logest.
Combined contraceptives affect the metabolism of cyclosporine , leading to a decrease in its plasma concentration. Taking estrogen-progestin drugs requires adjustment of the dosage of glucose-lowering drugs, as well as indirect anticoagulants.
Logest's analogs
Level 4 ATC code matches:
Ovidon
Rigevidon
Non-Ovlon
Mercilon
Yarina Plus
Yarina
Miniziston 20 fem
Novinet
Microgynon
Janine
Lindineth
Cyclo-Proginova
Regulon
Midiana
Belara
Femoden
Jess Plus
Jess
Zoely
Artisia, Lindinet 20, Lindinet 30, Femoden.
Analogues Artisia and Lindinet 20 have the same composition. The preparations Lindinet 30 and Femoden contain gestodene 75 mcg, ethinyl estradiol 30 mcg.
Reviews about Logest
Reviews from women about Logest indicate that the use of the drug is highly reliable. Tolerability of the drug is assessed as very good. Women note that taking the drug does not affect body weight even with long-term use. There is also no androgenic effect on skin and hair growth.
Reviews from doctors indicate the positive clinical characteristics of the drug. Reducing the dose of hormones significantly reduces the number of side effects. At the same time, the drug has high bioavailability , which makes it possible to predict its level in the blood and use it at a lower dose. There is a lower risk of intermenstrual bleeding when taking products containing gestodene. Doctors prescribe it to regulate the menstrual cycle. The drug is effective for heavy, prolonged and painful menstruation. With its use, painful periods are observed less frequently, the intensity of discharge decreases, and the risk of anemia decreases.
Analyzing reviews of Logest on forums, we can conclude that Logest is recommended for women who are starting to “get acquainted” with oral contraceptives. It is used when high-dose drugs are poorly tolerated. In addition, it is used for the treatment and prevention of many gynecological diseases.
Among the disadvantages of the drug, it is noted that it cannot be taken with many medications (even with analgin), the appearance of cellulite . For many, an important point is the price. The high price of this drug is considered a disadvantage.
Special instructions for the use of the drug Logest
If any of the following conditions/risk factors are present, the benefits of using COCs should be assessed against the possible risks, taking into account the individual characteristics of each patient and discussed with her before she decides to take COCs. If any of the following conditions or risk factors become worse, worse, or occur for the first time, it is recommended that you consult your doctor. The doctor must decide whether to stop using the COC. Circulatory disorders Based on the results of epidemiological studies, there is an association between the use of COCs and an increased risk of venous and arterial thrombotic and thromboembolic diseases, such as myocardial infarction, stroke, deep vein thrombosis and pulmonary embolism. These conditions occur rarely. Venous thromboembolism (VTE), manifested as venous thrombosis and/or pulmonary embolism, can occur with the use of any COC. The risk of venous thromboembolism is highest during the 1st year of COC use. The incidence of VTE in women taking low-dose estrogen oral contraceptives (≤0.05 mg ethinyl estradiol) is up to 4 cases per 10,000 women/year compared with 0.5–3 cases per 10,000 women/year in women not using oral contraceptives. The incidence of VTE associated with pregnancy is 6 cases per 10,000 women/year. Thrombosis of other blood vessels, such as arteries and veins of the liver, kidneys, mesenteric vessels, cerebral or retinal vessels, has been extremely rarely reported in women using COCs. There is no consensus regarding the connection between these complications and the use of COCs. Symptoms of venous or arterial thrombotic/thromboembolic events or stroke may include: unilateral lower extremity pain or swelling; sudden severe chest pain radiating to the left arm; sudden shortness of breath; sudden onset of cough; any unusual, severe, prolonged headache; sudden decrease or complete loss of vision; diplopia; speech impairment or aphasia; vertigo; collapse with or without partial epileptic seizure; weakness or very severe sudden numbness of one side or one part of the body; motor impairment; acute abdomen syndrome. Factors that increase the risk of venous or arterial thrombotic/thromboembolic events or stroke:
- age;
- tobacco smoking (in combination with heavy smoking and with age, the risk increases, especially in women over 35 years of age);
- family history (for example, cases of venous or arterial thromboembolism in siblings or parents at a relatively early age).
If a hereditary tendency is suspected, before making a decision on the use of any PDA, the woman should be referred for consultation to an appropriate specialist:
- obesity (body mass index more than 30 kg/m2);
- dyslipoproteinemia;
- hypertension;
- migraine;
- heart valve pathology;
- atrial fibrillation;
- prolonged immobilization, radical surgical interventions, any surgical operations on the lower extremities, significant injuries.
In these cases, it is recommended to stop using the PDA (for planned operations at least 4 weeks before they are performed) and not restore it earlier than 2 weeks after complete remobilization. There is no consensus regarding the possible role of varicose veins and superficial thrombophlebitis in the development of venous thromboembolism. It is necessary to take into account the increased risk of thromboembolism in the postpartum period. Other diseases that may be associated with serious circulatory disorders include diabetes mellitus; systemic lupus erythematosus; hemolytic uremic syndrome; chronic inflammatory bowel disease (Crohn's disease or ulcerative colitis) and sickle cell anemia. An increased incidence of migraine or its exacerbation during the period of use of COCs (which may be a harbinger of cerebrovascular accident) may require urgent cessation of COC use. Biochemical indicators characteristic of a hereditary or acquired tendency to venous or arterial thrombosis include: resistance to CRP, hyperhomocysteinemia, antithrombin III deficiency, protein C deficiency, protein S deficiency, antiphospholipid antibodies (anticardiolipin antibodies). When analyzing the risk/benefit ratio, the physician should take into account that adequate treatment for the conditions mentioned above can reduce the associated risk of thrombosis, and also that the risk of thrombosis associated with pregnancy is higher than with the use of COCs in low doses (≤0.05 mg ethinyl estradiol). Tumors The most important risk factor for the development of cervical cancer is the persistence of papillomavirus. Some epidemiological studies indicate an additional increase in this risk with long-term use of COCs, however, this statement is controversial because the extent to which the study results account for concomitant risk factors, such as cervical smears and sexual behavior, including the use of barrier methods of contraception, is not clear. . The results of a meta-analysis based on data from 54 epidemiological studies indicate a slight increase in the relative risk (RR = 1.24) of developing breast cancer in women using COCs. This increased risk gradually disappears within 10 years of stopping taking COCs. Because breast cancer is rarely diagnosed in women under 40 years of age, the increase in breast cancer diagnosis among current or recent COC users is small relative to the overall risk of breast cancer. The results of these studies do not provide evidence of a causal relationship. The increased risk may be due to both earlier diagnosis of breast cancer in women using COCs, the biological effects of COCs, or a combination of both factors. There is a tendency that breast cancer detected in women who have ever taken COCs is clinically less severe than in those who have never taken COCs. In isolated cases, benign, and even less often, malignant liver tumors were noted in women using COCs, which sometimes led to the development of life-threatening intra-abdominal bleeding. If there are complaints of severe pain in the epigastric region, liver enlargement, or signs of intra-abdominal bleeding, the differential diagnosis should take into account the possibility of a liver tumor in women taking COCs. Other conditions Women with hypertriglyceridemia or a family history of this disorder are at risk of developing pancreatitis when using COCs. Although slight increases in blood pressure have been reported in many women taking COCs, clinically significant increases in blood pressure are rare. However, if prolonged clinically significant hypertension (arterial hypertension) occurs during the period of taking COCs, then sometimes it is more appropriate to discontinue the COC and direct treatment to hypertension (arterial hypertension). The occurrence or exacerbation of the following diseases has been reported during pregnancy and with the use of COCs, but their relationship with the use of COCs has not been conclusively established: jaundice and/or pruritus associated with cholestasis, gallstone formation, porphyria, systemic lupus erythematosus, hemolytic uremic syndrome, Sydenham's chorea, herpes of pregnancy, hearing loss associated with otosclerosis. In acute or chronic liver dysfunction, it may be necessary to stop taking COCs until liver function tests return to normal. If cholestatic jaundice relapses, which first occurred during pregnancy or previous use of sex hormones, taking COCs should be discontinued. Although COCs may affect peripheral insulin resistance and glucose tolerance, there are no data regarding the need to change the therapeutic regimen in women with diabetes mellitus taking low-dose COCs (containing ≤0.05 mg ethinyl estradiol). However, women with diabetes should be closely monitored while taking COCs. Crohn's disease and ulcerative colitis may be associated with COC use. Chloasma can sometimes occur, especially in women with a history of chloasma during pregnancy. Women prone to chloasma should avoid exposure to direct sunlight or ultraviolet radiation while taking COCs. Medical examination Before starting or reinstating the use of the drug Logest, a full medical examination should be performed and the patient’s medical history should be studied in detail, taking into account contraindications (see CONTRAINDICATIONS) and warnings (see APPLICATION). When using COCs, it is recommended to conduct periodic examinations, which is very important, since contraindications (transient circulatory disorders, etc.) or risk factors (for example, a family history of venous or arterial thrombosis) may first appear during the period of taking COCs. The frequency and nature of these examinations should be based on the standards of medical practice, taking into account the individual characteristics of each woman, however, special attention is paid to the examination of the pelvic organs, including a standard analysis of cytology of the cervix, abdominal organs, mammary glands, and determination of blood pressure. The patient must be warned that oral contraceptives do not protect against HIV infection (AIDS) and other sexually transmitted diseases. Decreased effectiveness The effectiveness of combined oral contraceptives may be reduced if a pill is missed, gastrointestinal dysfunction or other medications are used. Cycle control When taking oral contraceptives, intermenstrual bleeding (spotting or breakthrough bleeding) may occur, especially during the first few months of treatment. Taking this into account, examination in the event of any intermenstrual bleeding should be carried out only after a period of adaptation of the body to the drug, which is approximately 3 cycles. If menstrual irregularities continue or recur after several normal cycles, non-hormonal causes of bleeding should be considered and appropriate investigations should be carried out to exclude the presence of tumors and pregnancy. Diagnostic measures can include curettage. Some women may not experience menstrual bleeding during a break from taking the drug. There is a low chance of pregnancy when you take COCs as directed. However, if the contraceptive was taken irregularly or if menstrual-like bleeding is absent for 2 cycles, pregnancy must be excluded before continuing to take the COC. Pregnancy and lactation The drug is contraindicated for use during pregnancy. If pregnancy occurs during the use of the drug Logest, it is necessary to stop taking it. However, research results do not indicate an increased risk of congenital pathologies in children born to mothers who took COCs during pregnancy, nor do they indicate the existence of a teratogenic effect when unintentionally taking COCs in early pregnancy. PDAs can affect lactation, since under their influence the amount of breast milk can decrease and also change its composition. With this in mind, COCs are not recommended for use while breastfeeding. The active substances that make up the drug and/or their metabolites are excreted in small quantities in breast milk, but there is no data on their negative effect on the health of the infant. Impact on the ability to drive vehicles and operate machinery No impact was noted.
Logest price, where to buy
You can buy it in pharmacies in Moscow and other cities of Russia. For Logest contraceptive pills, the price ranges from 545 rubles. up to 712 rub. for 21 tablets
- Online pharmacies in RussiaRussia
- Online pharmacies in UkraineUkraine
- Online pharmacies in KazakhstanKazakhstan
ZdravCity
- Logest dragee 63 pcs. Bayer Weimar GmbH and Co. KG/Bayer AG
RUB 2021 order - Logest tablets 21 pcs. Bayer Weimar GmbH and Co.KG
RUR 822 order
Pharmacy Dialogue
- Logest (table no. 21) Bayer
RUR 791 order
- Logest (tablet. no. 21x3) Bayer
RUB 1,955 order
show more
Pharmacy24
- Logest No. 21 tablets Delpharm Lille S.A.S., France
200 UAH.order
PaniPharmacy
- Logest tablets Logest etc. No. 21 France, Delpharm Lille
221 UAH order
show more