"INFANRIX HEXA" - vaccination against diphtheria, tetanus, whooping cough, polio, hepatitis B, and Haemophilus influenzae type b (Hib)
Combined vaccine, hexavalent
Manufacturer: GlaxoSmithKline Biologicals, Belgium.
Protects against diseases: diphtheria, tetanus, whooping cough, polio, hepatitis B and Haemophilus influenzae type b.
Used: in children in the first and second year of life.
Included in the national vaccination calendar.
Benefits of the Infanrix Hexa vaccine
- Minimal number of side effects compared to DPT.
- A protective effect against three types of polioviruses is observed in 91% of vaccinated children aged 4-8 years, against diphtheria and tetanus in 64.7% of children, against whooping cough in 87% of children.
- Immunity against Hib is initiated in 96% of vaccinated children.
- The protective effect against hepatitis B persists in 48% at the age of 11-12 years.
- Available clinical data from more than 10 years of vaccine experience indicate that Infanrix Hexa, as a primary and booster vaccination, is a safe and useful option for providing protection against common childhood diseases, diphtheria and tetanus.
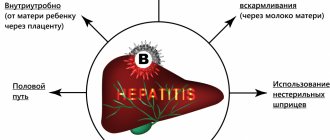
- The vaccine indirectly protects against hepatitis D, which does not occur in the absence of hepatitis B.
Description of the drug
The Infanrix Hexa vaccine is one of the most modern combination vaccines, which allows you to simultaneously protect a child from six dangerous infections. The vaccine has been successfully used in Europe for about 10 years and meets all WHO requirements for the production of biological substances.
Infanrix Hexa is an adsorbed acellular (acellular) diphtheria-tetanus-pertussis vaccine, inactivated polio vaccine, recombinant hepatitis B vaccine and a vaccine for the prevention of Haemophilus influenzae type b.
The Infanrix Hexa vaccine contains diphtheria and tetanus toxoid antigens, components of the cell wall of the causative agent of whooping cough, inactivated polio virus types 1,2,3, capsular polysaccharides of Haemophilus influenzae type b, and genetically engineered HbsAg (hepatitis B antigen).
Using the most modern technologies in the production of Infanrix Hexa, it was possible to reduce the number of protein molecules by 30 times (compared to whole-cell vaccines - DPT) without loss of immunogenicity, which has been proven in numerous clinical studies for each component. With the same effectiveness as whole cell vaccines (DTP), the number of adverse reactions after vaccination with Infanrix Hexa is significantly reduced. The presence of six components in one vaccine allows you to minimize the number of injections, thereby significantly reducing the number of complications and discomfort in the child.
Contraindications
Only a doctor can decide whether Infanrix Hexa is suitable for vaccination
Vaccination with Infanrix Hexa is contraindicated if there is a history of an allergic reaction to any component of the vaccine, as well as in the following cases:
- Hypersensitivity to the active substances or to any excipients.
- Hypersensitivity after previous vaccination against diphtheria, tetanus, whooping cough.
- Case of encephalopathy within 7 days after any pertussis vaccination.
Vaccination scheme
The primary vaccination regimen consists of three doses of 0.5 ml (vaccine administration at the age of 2–3–4 months; or 3–4–5 months; or 2–4–6 months) or two doses (vaccine administration at the age of 3 ; 5 months). There should be an interval of at least 1 month between doses. Infanrix Hexa administered according to the EPI (Expanded Program on Immunization; 6–10–14 weeks of age) schedule can only be used if the vaccine recipient has received a dose of hepatitis B vaccine at birth.
After vaccination with two doses (eg at 3–5 months of age) of Infanrix Hexa, a booster dose should be given at least 6 months after the last primary dose, preferably between 11 and 13 months of age. After vaccination with three doses (eg, 2–3–4 months; 3–4–5 months; 2–4–6 months) of Infanrix Hexa, a booster dose should be given at least 6 months after the last primary dose, preferably under the age of 18 months.
Possible side effects
The most commonly observed side effects are:
- decreased or complete loss of appetite
- the injection site may become red and painful, and swelling may occur
- the child may become restless and irritable and cry for a long time
- increase in body temperature to 39.5 degrees
More rare manifestations of a reaction to the vaccine:
- vomiting and diarrhea
- the appearance of thickening and redness at the vaccine site
- allergic reaction
Come get vaccinated at VIRILIS. A full range of vaccines for children and adults, family vaccinations - at a special price!
pharmachologic effectVaccine against diphtheria, tetanus and whooping cough. Infanrix® Hexa contains diphtheria toxoid, tetanus toxoid, three purified pertussis antigens [pertussis toxoid (PTA), filamentous hemagglutinin (FHA) and outer membrane protein (pertactin) with a molecular weight of 69 kDa], purified major surface antigen (HBsAg) of the hepatitis B virus and purified capsular polysaccharide of Haemophilus influenzae type b, covalently bound to tetanus toxoid, adsorbed on aluminum salts. The vaccine also contains three types of inactivated polioviruses (type 1 - Mahoney strain, type 2 - MEF-1 strain, type 3 - Saukett strain).
Tetanus and diphtheria toxoids are produced by treating purified Corynebacterium diphtheriae and Clostridium tetani toxins with formaldehyde. Acellular pertussis vaccine components are obtained from Bordetella pertussis culture by extraction and purification, followed by detoxification with glutaraldehyde and formaldehyde of pertussis toxin, and formaldehyde of filamentous hemagglutinin and pertactin.
Hepatitis B virus surface antigen is produced by a culture of yeast cells (Saccharomyces cerevisiae), obtained by genetic engineering and having a gene encoding the main surface antigen of the hepatitis B virus. HBsAg isolated from yeast cells is purified using sequentially applied physicochemical methods. HBsAg, in the absence of chemical treatment, spontaneously transforms into spherical particles with a diameter of 20 nm containing non-glycosylated HBsAg polypeptides and a lipid matrix consisting mainly of phospholipids. Studies have shown that these particles have properties characteristic of natural HBsAg.
Three types of polioviruses grown on VERO cells are purified and inactivated with formaldehyde.
Hib polysaccharide is prepared from Haemophilus influenzae type b strain 20.752. After activation with cyanogen bromide and derivatization with adipic acid hydrazide, it is combined with tetanus toxoid by the carbodiimide method. After purification and conjugation, the polysaccharide is adsorbed onto aluminum salts, then lyophilized in the presence of lactose as a stabilizer.
Infanrix® Hexa meets WHO requirements for the production of biological substances, diphtheria, tetanus, pertussis and combination vaccines, as well as recombinant hepatitis B vaccines, inactivated polio vaccines and Haemophilus influenzae type b conjugate vaccines.
The results obtained in clinical studies for each vaccine component are presented in Tables 1 and 2.
Table 1. Percentage of participants with antibody titer ? borderline value 1 month after the course of primary vaccination with the Infanrix® Hexa vaccine.
Antibodies (borderline value)
| 2 doses | 3 doses | ||||
| 3-5 months n= 530 (4 studies) | 2-3-4 months n= 196 (2 studies) | 2-4-6 months n= 1693 (6 studies) | 3-4-5 months n= 1055 (6 studies) | 6-10-14 weeks n= 265 (1 study) | |
| % | % | % | % | % | |
| Anti-diphther. (0.1 IU/ml) # | 98.0 | 100.0 | 99.8 | 99.7 | 99.2 |
| Anti-pillar (0.1 IU/ml)# | 100.0 | 100.0 | 100.0 | 100.0 | 99.6 |
| Anti-CT (5 EL.E/ml) | 99.5 | 100.0 | 100.0 | 99.8 | 99.6 |
| Anti-PHA (5 EL.U/ml) | 99.7 | 100.0 | 100.0 | 100.0 | 100.0 |
| Anti-PRN (5 EL.U/ml) | 99.0 | 100.0 | 100.0 | 99.7 | 98.9 |
| Anti-HBs (10 mIU/ml) # | 96.8 | 99.5 | 98.9 | 98.0 | 98.5* |
| Anti-polyotype 1 (1/8 dilution)# | 99.4 | 100.0 | 99.9 | 99.7 | 99.6 |
| Anti-polyotype 2 (1/8 dilution) # | 96.3 | 97.8 | 99.3 | 98.9 | 95.7 |
| Anti-polyotype 3 (1/8 dilution)# | 98.8 | 100.0 | 99.7 | 99.7 | 99.6 |
| Anti-PRP (0.15 µg/ml) # | 91.7 | 96.4 | 96.6 | 96.8 | 97.4 |
* - in the subgroup of children who did not receive the hepatitis B vaccine at birth, 77.7% of children had a titer of anti-HBs antibodies (? 10 mIU/ml).
# — a borderline value indicates the presence of protection.
EL.E - ELISA units (enzyme-linked immunosorbent assay, ELISA).
PRP - polyribosyl rebitol phosphate.
Table 2. Percentage of participants with antibody titer ? borderline value 1 month after revaccination with the Infanrix® Hexa vaccine.
| Antibodies (borderline value) | Revaccination at 11 months after primary vaccination at 3-5 months n = 532 (3 studies), % | Revaccination in the second year of life after three-dose primary vaccination n = 2009 (12 studies), % |
| Anti-diphther. (0.1 IU/ml)# | 100.0 | 99.9 |
| Anti-column (0.1 IU/ml)# | 100.0 | 99.9 |
| Anti-CT (5 EL.E/ml) | 100.0 | 99.9 |
| Anti-PHA (5 EL.U/ml) | 100.0 | 99.9 |
| Anti-PRN(5 EL.U/ml) | 99.2 | 99.5 |
| Anti-HBs(10 mIU/ml) # | 98.9 | 98.4 |
| Anti-poliotype1 (1/8 dilution)# | 99.8 | 99.9 |
| Anti-poliotype2 (1/8 dilution)# | 99.4 | 99.9 |
| Anti-poliotype3 (1/8 dilution)# | 99.2 | 99.9 |
| Anti-PRP (0.15 µg/ml)# | 99.6 | 99.7 |
# — a borderline value indicates the presence of protection.
Since the immune response to pertussis antigens after administration of the Infanrix® Hexa vaccine is equivalent to that after the administration of the Infanrix® vaccine, the protective activity of both vaccines is equivalent.
The protective effect of the pertussis component of the Infanrix® Hexa vaccine against typical pertussis (as defined by WHO) (≥21 days of paroxysmal cough) was proven in the following studies:
- a prospective, blinded study involving household exposure to pertussis, conducted in Germany (vaccination schedule 3, 4 and 5 months). Based on data collected during follow-up contacts with families in which the first case of typical whooping cough was reported, the protective effect of the vaccine was 88.7%;
- National Institute of Health-sponsored study conducted in Italy (vaccination schedule 2, 4 and 6 months). The vaccine was found to be 84% effective. Follow-up in the same cohort found that demonstrated efficacy was maintained for up to 60 months after completion of primary vaccination, without an additional dose of pertussis vaccine.
Long-term follow-up results from Sweden indicate that acellular pertussis vaccines are highly effective in children when given at 3 and 5 months of age, with a booster dose at approximately 12 months.
The protective level of antibodies against hepatitis B after administration of Infanrix® Hexa did not differ from the level recorded in a parallel group receiving monovalent hepatitis B vaccine.
The effectiveness of the Hib component (when coadministered with DTPa, or DTPa-IPV or DTPa-HBV-IPV) has been and continues to be studied in a large post-marketing observational study conducted in Germany. During the 4.5-year follow-up period, the effectiveness of DTPa/Hib or DTPa-IPV/Hib vaccines was 96.7% for primary vaccination and 98.5% for booster doses (regardless of primary immunization).
During the three-year follow-up period, the efficacy of hexavalent vaccines was 92.8% for the completed primary series and 100% for the booster dose.
Indications for use of the drug INFANRIX® HEXA
- primary and additional vaccination of children against diphtheria, tetanus, whooping cough, hepatitis B, polio and meningitis caused by Haemophilus influenzae type b.
Dosage regimen
Infanrix® Hexa should be administered deep intramuscularly.
Primary vaccination
The primary vaccination regimen consists of three doses of 0.5 ml (at 2, 3, 4 months; at 3, 4, 5 months; at 2, 4, 6 months) or two doses (at 3, 5 months). The interval between doses should be at least 1 month. It is possible to immunize a child according to the schedule of 6, 10, 14 weeks only if a dose of hepatitis B vaccine was administered at birth.
Local hepatitis B immunization guidelines should be followed. Where a dose of hepatitis B vaccine is given at birth, Infanrix® Hexa can replace additional doses of hepatitis B vaccine starting at 6 weeks of age. If a second dose of hepatitis B vaccine is required before this age, monovalent hepatitis B vaccine should be used.
Additional vaccination
After vaccination with two doses (for example, at 3 and 5 months) of the Infanrix® Hexa vaccine, an additional dose of the vaccine should be administered no earlier than 6 months after the first vaccination (preferably between 11 and 13 months).
After vaccination with three doses (for example, at 2, 3, 4 months; at 3, 4, 5 months; at 2, 4, 6 months) Infanrix® Hexa, no earlier than 6 months after the first vaccination (preferably before 18 months) , an additional dose of vaccine may be given.
Additional vaccinations should be carried out in accordance with official recommendations.
Infanrix® Hexa can be considered as a vaccine for additional vaccination if its composition complies with official recommendations.
Rules for using the vaccine
The syringe is shaken vigorously until a homogeneous cloudy white suspension is obtained. The syringe and vial must be carefully inspected for foreign particles or changes in the declared appearance. In case of any discrepancy, the vaccine must be destroyed.
A ready-to-use vaccine is prepared by adding the contents of a syringe to a vial containing Hib lyophilisate. It is recommended to remove the bottle from the refrigerator 5 minutes before dilution, because in this case, a degree of elasticity of the cork is ensured at which the risk of rubber particles coming off is minimal. The reconstituted vaccine is a slightly cloudier suspension than the contents of the syringe before reconstitution. This is normal and does not affect the quality of the vaccine. If other inconsistencies are found, the vaccine must be destroyed.
The vaccine should be administered immediately after recovery.
Side effect
The adverse reaction data below is based on information obtained from vaccination of more than 16,000 children.
After additional vaccination with Infanrix® Hexa, an increase in the frequency of local reactions and fever was noted compared to the primary course.
Determination of the frequency of adverse reactions: very often (? 10%, < 20%), often (from ? 1% to ? 10%), sometimes (from ? 0.1% to ? 1%), rarely (from ? 0.01% to < 0.1 %), very rare (<0.01%), including isolated reports.
From the digestive system: very often - loss of appetite; often - vomiting, diarrhea.
From the body as a whole: very often - anxiety, abnormal crying, irritability, increased body temperature from 38°C to 39.5°C; increase in body temperature more than 39.5°C.
Local reactions: very often - including pain, redness, swelling (? 50 mm); often - swelling at the injection site (> 50 mm) and compaction; sometimes - diffuse swelling of the limb into which the injection was made, sometimes affecting the adjacent joint.
Dermatological reactions: often - itching; rarely - rash; very rarely - dermatitis, urticaria.
From the respiratory system: sometimes - upper respiratory tract infections, cough; rarely - bronchitis.
From the side of the central nervous system: sometimes - nervousness, drowsiness; very rarely - convulsions (with or without fever).
Post-marketing data
Very rare: enlarged lymph nodes, thrombocytopenia, allergic reactions (including anaphylactic and anaphylactoid reactions), angioedema, collapse or shock states (hypotonic or hyporesponsive), apnea, swelling of the limb into which the injection was made (edema subsides on average within 4 days) .
Isolated reports (<0.01%) in children under 2 years of age (causal relationship with vaccine use not established: paralysis, neuropathy, Guillain-Barré disease (acute primary idiopathic polyradiculoneuritis), encephalopathy, encephalitis, meningitis, serum sickness, neuritis, hypotension , vasculitis, erythema multiforme, arthritis, muscle weakness.
Contraindications to the use of INFANRIX® HEXA
- hypersensitivity to the components of the drug;
- hypersensitivity reaction to previous administration of vaccines against diphtheria, tetanus, whooping cough, hepatitis B or polio;
— encephalopathy of unknown etiology within 7 days after the previous vaccination with the pertussis component. In this case, pertussis vaccination should be interrupted and immunization should be continued with diphtheria-tetanus vaccine, hepatitis B vaccine, inactivated polio vaccines and Haemophilus influenzae type b vaccine.
Use of INFANRIX® HEXA during pregnancy and breastfeeding
Infanrix® Hexa is not intended for use in adults.
special instructions
The use of Infanrix® Hexa, like other vaccines, should be postponed in children with acute febrile conditions. The presence of a mild infectious disease is not a contraindication for vaccination.
Vaccination must begin with a study of the medical record (particular attention is paid to previous vaccinations and cases of adverse events) and medical examination.
If any of the following phenomena occurred after the use of a pertussis-containing vaccine, the decision to continue taking vaccines with a pertussis component must be carefully weighed:
- Body temperature ? 40°C for 48 hours, in the absence of other reasons;
— collapse or shock state (hypotonic or hyporesponsive) within 48 hours after vaccination;
- continuous crying, lasting more than 3 hours, within 48 hours after vaccination;
- convulsions with or without fever within 72 hours after vaccination.
In some circumstances, such as a high incidence of whooping cough, the potential benefits of vaccination may outweigh the risks.
As with other injectable vaccines, emergency supplies should always be available when administering Infanrix® Hexa in the event of the rare occurrence of anaphylactic reactions.
Infanrix® Hexa should be used with caution in case of thrombocytopenia or blood coagulation disorders, since bleeding may occur during intramuscular administration.
Under no circumstances should the vaccine be administered intravenously or intradermally.
Infanrix® Hexa contains traces of neomycin and polymyxin. The vaccine should be used with caution in patients with known hypersensitivity to these antibiotics.
The HBV component of the vaccine does not prevent infections caused by hepatitis A, C, E and other pathogens that cause liver disease. However, protection against hepatitis D (caused by the delta agent) is expected, since hepatitis D cannot occur in the absence of hepatitis B virus.
The Hib component of the vaccine does not protect against diseases caused by other types of Haemophilus influenzae, or meningitis caused by other microorganisms.
A protective level of immune response may not be achieved in all patients.
A history of febrile seizures, as well as a family history of seizures or sudden infant death are not contraindications for the use of Infanrix® Hexa. Vaccine recipients with a history of febrile seizures should be closely monitored because possible adverse reactions may occur within 2 or 3 days after vaccination.
HIV infection is not a contraindication to the use of Infanrix® Hexa. The expected level of immune response may not be achieved in immunocompromised patients.
Because Hib capsular polysaccharide is excreted in the urine, a urine test for Hib infection will be positive within 1-2 weeks after vaccination. To confirm the disease during this period, it is necessary to use other analytical methods.
Limited clinical data (169 infants) have been obtained regarding the feasibility of vaccination of Infanrix® Hexa in premature infants. A low level of immune response may occur, and the level of clinical protection remains unknown.
If shortness of breath occurs, monitoring of respiratory function is necessary for 48-72 hours after primary immunization, especially in premature infants (born at ≥28 weeks of gestation) and in cases where they already have a history of respiratory failure. Because The potential benefit of vaccination in this group of children is high; immunization should not be refused or postponed.
Overdose
There is no data on overdose of the Infanrix® Hexa vaccine.
Drug interactions
At the moment, there is insufficient data on the effectiveness and safety of vaccination when using Infanrix® Hexa together with the measles, mumps and rubella vaccine, therefore it is necessary to ensure an interval of at least one month between their administration.
The expected level of immune response may not be achieved in patients receiving immunosuppressive therapy.
Pharmaceutical incompatibility
Infanrix® Hexa cannot be mixed with other vaccines in the same syringe.
Conditions for dispensing from pharmacies
The drug is available with a prescription.
Storage conditions and periods
The drug should be stored out of the reach of children, protected from light at a temperature of 2° to 8°C; do not freeze. Shelf life: 3 years. The date of last use should be considered the last day of the specified month.
Frozen vaccine must be destroyed.
For children
It is recommended to start injections from infancy in accordance with the vaccination calendar approved by the Ministry of Health.
From 3 to 6 months they take a course that includes a complete package of antibodies:
- Diphtheria.
- Whooping cough.
- Tetanus.
- Polio.
- Haemophilus influenzae infection.
- Hepatitis group B.
First, a specialist, most often an immunologist, checks the child for diseases, general health, possible contraindications, and measures the temperature.
It is vital to monitor the general condition of the child a week before vaccination and the same period after. It is worth paying great attention to the quality of the doctor’s training, but you should not bombard him with questions and talk “hand in hand”. He is also a person and concentrates on his task, especially when it comes to vaccines for a baby.
The quality of the supplied packaging is inspected in front of the patient. The suspension is stored in the refrigerator and will be taken out just a few minutes before the injection. It is important that everything is hermetically sealed, does not contain foreign substances, and “access to manpower is blocked” (no holes). Of course, the drug must remain fresh.
The doctor knows exactly where and how to inject the liquid. Everything is injected intramuscularly into the middle third of the thigh. A secondary procedure is done in the same area, but on the other leg.
A mandatory requirement is to stay in the clinic for 30 minutes. This way, the child will remain close to medical care if a severe sharp deterioration in his condition suddenly occurs. The doctor will monitor the body’s reaction and give further recommendations to the parent on how to care for their child and schedule the next session for the primary immunization.
MANUFACTURER
GlaxoSmithKline biologicals with a. / GlaxoSmithKlme Biologicals sa Rue de l'Enstitut, 89.1330 Rixensart, Belgium / Rue de I'lnslitut, 89.1330 Rixensart, Belgium
NAME AND ADDRESS OF LEGAL ENTITY. IN WHOSE NAME THE REGISTRATION CERTIFICATE IS ISSUED
CJSC GlaxoSmithKline Trading 119180, Moscow. Yakimanskaya embankment d 2 For more information contact:
CJSC GlaxoSmithKline Trading: 121614 Moscow, Krylatskaya st., 17, bldg. 3, floor S Business Park “Krylatskie Hills” Tel.: (495) 777-89-00; Fax
Interaction
Infanrix Hexa vaccine has the remarkable ability to be combined with other medications for immunization. But it cannot be combined with BCG. In this case, you can even complete the course of vaccination with it or, conversely, with other means (DTP, Pentaxim)
It is well complemented by antiallergic drugs. They will reduce the possible risk of developing pathology after the injection. Sometimes suppositories or suspensions with a sedative and analgesic effect are used.
For those suffering from neurological diseases, as a preparation for Infarix Gexa, drink:
- diuretics;
- sedatives;
· anticonvulsants.
There are nuances that can disrupt the usual course of the procedure. If the vaccination before the aforementioned manufacturer was against hepatitis B, then to continue you should choose the regular Infanrix or only with three toxoids. Sometimes the Hib component should not be mixed (it is included and the doctor dilutes the dried material in a test tube).