Sodium bicarbonate, sodium bicarbonate, sodium bicarbonate, baking soda are the names of the same chemical compound, known to every person as “baking soda.” It is a good leavening agent for dough. Soda solution can relieve toothache. But this is not the entire range of applications of this “miracle” substance. In fact, it is difficult to do without sodium bicarbonate in everyday life, in cooking, in medicine, and in many other areas of activity.
History of creation
Baking soda has been used in baking since ancient times. It was found by archaeologists during excavations of caves of the 1st-2nd centuries BC. Then it was extracted from the ash of seaweed or found in the form of a mineral. This chemical compound was actively used in ancient Egypt.
For the first time, the chemical formula of the compound - NaHCO3 - was established by the French scientist Henri de Monceau. Thanks to this discovery, baking soda began to be produced synthetically, which significantly reduced its cost and expanded its range of uses. Since the discovery of the formula, methods for its synthesis have constantly changed, improved, and become more economically profitable.
Methods of obtaining
Content:
- History of creation
- Chemical properties
- Beneficial features
- Possible harm
- Medical use
- Use in cooking
- Application on the farm
- Use in cosmetology
- Other uses
- Industrial use
- How to select and store
The first method of industrially producing sodium carbonate was to dissolve rock salt in water, mix the solution with limestone and charcoal, and then heat it in a furnace. However, as it turned out, the output was not baking soda, but soda ash. In addition, this activity left a lot of toxic waste (calcium sulfide and hydrogen chloride), so it was quickly abandoned.
Today, baking soda is produced in two ways - “dry” and “wet”, each of which is based on the carbonization reaction (enrichment of the solution with carbon dioxide).
Types of soda
From a chemical point of view and area of application, there are several types of soda: baking soda (drinking soda), ash soda (linen soda) and caustic soda (sodium hydroxide).
Production
People have been familiar with baking soda for a very long time. It was mined on some dry lakes, where sodium salts fell ashore in white snowdrifts.
Natural raw materials were mined in two types:
- in the form of sodium salts (carbonates and bicarbonates),
- in groundwater containing high concentrations of sodium carbonate.
The well-known chemical formula NaHCO3 is obtained not naturally, but by a chemical laboratory method. It's called ammonium chloride.
The method for producing sodium bicarbonate is ammonium chloride, developed in the 19th century by the chemist E. Solvay. The method is still relevant today.
The industrial method of soda production was first used by the French scientist N. Leblanc, isolating sodium carbonate from rock salt, resulting in soda ash. Another Frenchman O.J. Fresnel passed rock salt through an ammonia solution and carbon dioxide. This is how a chemical method was invented to form bicarbonate or baking soda.
The Belgian chemist E. Solvay improved the method, making it simple and cheap. Using the Solvay method, they began to produce (and still produce) not only soda ash, but also baking soda.
Chemical properties
Sodium bicarbonate is a weak acid salt of carbonic acid. They are small colorless crystals, which, when the temperature rises to 50-60°C, begin to “give up” a molecule of carbon dioxide, gradually decomposing to sodium carbonate (soda ash).
Reacts with acids to form salts (chloride, acetate, sodium sulfate) and carbonic acid, which instantly breaks down to water and carbon dioxide. The powder is poorly soluble in water and is easily separated by filtration.
Receipt
In industry, sodium bicarbonate is produced by the ammonia-chloride method. Carbon dioxide is passed under pressure into a concentrated solution of sodium chloride, saturated with ammonia. During the synthesis process, two reactions occur:
NH3 + CO2 + H2O → NH4HCO3 NH4HCO3 + NaCl → NaHCO3↓ + NH4Cl
Sodium bicarbonate is slightly soluble in cold water, and it is separated from the cooled solution by filtration, and from the ammonium chloride solution obtained after filtration, ammonia is again obtained, which is returned to production again:
2NH4Cl + Ca(OH)2 → 2NH3↑ + CaCl2 + 2H2O
Beneficial features
The benefits of sodium bicarbonate come from its alkaline pH. It is the ability to react with acids and alkalize the environment that underlies the following beneficial properties of baking soda:
- acid neutralizing;
- antiseptic;
- anti-inflammatory;
- antipruritic;
- drying;
- antifungal;
- sputum thinner;
- softening and whitening skin.
Such a variety of useful properties allows this compound to be used in folk and traditional medicine to treat many diseases and normalize human well-being in various pathological and physiological conditions.
New research by scientists on treatment with soda
- In April 2021, a publication by American scientists from Augusta University appeared (in the Journal of Immunology) about the possibility of using baking soda for autoimmune diseases and in particular for rheumatoid arthritis. However, so far the experimental results have been confirmed only in laboratory conditions using rodents.
- At the end of May 2021, a study by scientists from the Ludwig Institute for Cancer Research (USA) was published on the use of sodium bicarbonate to increase the effectiveness of chemotherapy for malignant tumors. It has been established that ingestion of a solution of baking soda (again, we are talking only about rodents) helps to activate “dormant” cancer cells and increase their sensitivity to drugs and immunotherapy. Research into the effectiveness of sodium bicarbonate in the treatment of tumors will continue.
As you can see, the potential for the therapeutic effect of soda is quite high and this simple substance is used in a wide variety of branches of medicine. It remains only to wait for the final results of scientific research and clinical trials so that sodium bicarbonate can be used to improve health and at the same time not harm the body.
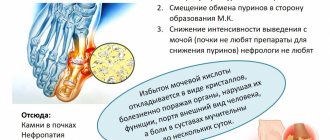
Possible harm
Baking soda should be consumed internally in limited quantities and according to strict indications. Bicarbonate crystals in large quantities are toxic to the mucous membrane of the digestive system and can cause severe irritation and allergic reactions.
It is not recommended to use solutions based on sodium bicarbonate for people suffering from erosive and ulcerative processes of the stomach and intestines, low stomach acidity and anacid gastritis.
If you regularly inhale carbon dioxide vapor or bicarbonate crystals, for example, in the production of soda, irritation of the respiratory mucosa may occur.
Frequent use of soda solution threatens persistent organic disorders of the digestive system. Alkalinization of gastric juice occurs, as well as a shift to the highly alkaline side of the intestinal contents.
When is soda contraindicated?
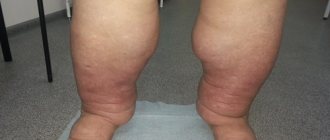
It is safe to take baking soda periodically and rarely as an emergency remedy for heartburn, colds, and dermatitis. But long-term use of sodium bicarbonate orally can lead to nausea and vomiting, worsening of stomach and duodenal ulcers, increased blood pressure, prolonged headaches, urinary retention, swelling, cramps and other adverse side effects. Therefore, before you decide to undertake any experiments on your health, be sure to consult with your doctor, because it may turn out that this substance is contraindicated for you personally.
Medical use
Sodium bicarbonate is widely used in medicine. At the same time, soda is used in various fields: dermatology, gastroenterology, cardiology, pulmonology, dentistry, toxicology, and ENT pathologies. Sodium bicarbonate helps against heartburn, nausea, motion sickness.
This substance is used internally in the form of a soda drink and externally in dry form, in the form of a paste or aqueous solution for rubdowns, lotions, and baths.
In dentistry
Rinsing the mouth with a solution of sodium bicarbonate relieves local inflammation, relieves toothache, strengthens gums, and eliminates unpleasant odor. Baking soda can be used as a toothpaste substitute to whiten teeth.
In gastroenterology
For nausea, make a strong soda solution (1 tablespoon per glass of water) and drink it slowly. For severe heartburn, it is recommended to dissolve a teaspoon of soda in a glass of water and drink. Thus, the patient’s condition improves for some time. However, it should be noted that if you experience frequent heartburn, you should consult a doctor and not treat yourself with soda at home. The habitual intake of an alkaline solution causes a neutralization reaction between hydrochloric acid and soda, resulting in the release of a lot of carbon dioxide, which causes bloating. The resulting carbon dioxide irritates the chemoreceptors of the stomach, thereby stimulating a reflex increase in the formation of gastric juice.
In cardiology
Hydrocarbonate baths help normalize blood pressure and heart rate, which is useful in case of interruptions in the functioning of the heart and blood vessels. Soda increases urination, which reduces the total volume of circulating blood. As a result, the pressure of the blood column on the walls of blood vessels decreases and blood pressure drops slightly.
Taking sodium bicarbonate solution orally with a sharp increase in blood pressure is a first aid remedy for a hypertensive crisis at home. If you drink a soda drink along with antihypertensive medications, the effect will increase.
In dermatology
Soap and soda baths and applications help get rid of fungal nail infections, as well as calluses and corns. A paste of baking soda and water should be used to treat areas of skin burns when exposed to acids, as well as areas of skin with sunburn. You need to moisten the areas of mosquito bites and other insects on the skin with water with baking soda dissolved in it. For severe itching, you can sprinkle the skin with dry powder.
If you have problems with the smell of sweat, treat your armpits with a soda solution. Bacteria and fungi that multiply in sweat and produce acids that cause an unpleasant odor will die. Sodium bicarbonate neutralizes these acids and exhibits a moderate antiseptic effect.
Hydrocarbonate-based foot baths are made for fungal diseases of the feet and nails. They also help soften rough heel skin before a pedicure. Hot baths of a strong solution of baking soda help with felon (purulent inflammation under the nail).
For ENT pathologies
Sodium bicarbonate, when released into viscous sputum, reacts with the acids contained in it. The resulting bubbles of carbon dioxide and water dilute the mucus, increase its quantity and make coughing easier.
To prepare an expectorant for tracheitis, laryngitis, bronchitis, as well as for severe cough, dilute a teaspoon of baking soda in 200 ml of warm milk. This elixir is drunk before bed. Instead of such a drink, you can do steam inhalations with soda. A tablespoon of powder is diluted in a liter of hot water and breathed over it. To enhance the effect, you can add a few drops of essential oils of eucalyptus, pine or rosemary to the solution. Gargling with a solution of salt and soda relieves inflammation of the tonsils with sore throat.
Intravenous administration of a sterile aqueous solution of sodium bicarbonate is often used in intensive care, infectious diseases departments and toxicology for poisoning and intoxication. metabolic acidosis.
Treatment with baking soda: indications
Let us list once again the main diseases for which sodium bicarbonate is used:
- Diseases in which metabolic acidosis syndrome occurs (diabetes mellitus, renal and liver failure, acute infectious diseases, severe poisoning, trauma, burns, postoperative period after complex abdominal surgical interventions) - sodium bicarbonate is administered intravenously
- Acute and chronic gastroduodenitis, reflux esophagitis, peptic ulcer of the stomach and duodenum - soda is used as a symptomatic remedy (for oral administration) to relieve heartburn, but is practically not used independently, but only as part of complex medications (Gaviscon et al. )
- Acute laryngotracheitis, bronchitis and other diseases of the upper respiratory tract, in which there is a cough with difficult to separate sputum (sodium bicarbonate is used orally and as a solution for inhalation)
- Seasickness and air sickness (baking soda taken orally or administered rectally for severe nausea and repeated vomiting)
- Inflammatory diseases of the oral cavity (stomatitis, tonsillitis, pharyngitis - in the form of a rinse solution)
- Topically, in pure form and in solution, sodium bicarbonate is used to reduce sweating of the feet and armpits during hyperhidrosis, as well as to relieve inflammation and irritation from insect bites and dermatitis.
Use in cooking
Sodium bicarbonate is also widely used in cooking. The ability of soda to release carbon dioxide when quenched with vinegar allows it to be used as a leavening agent. Slaked soda adds fluffiness to omelette and dough. You can quench soda with vinegar or add powder to sour cream or kefir dough. In the second case, lactic acid will play the role of vinegar.
Adding it to legume dishes allows you to reduce their cooking time. Using baking soda in a meat marinade can soften tough muscle fibers. Berry and fruit mousses, when a pinch of soda is added to them, become sweeter, and coffee and tea become more transparent and aromatic.
In order to get rid of nitrates in vegetables, they need to be soaked in a soda solution. Darkened potatoes can be lightened using the same method.
Features of treatment with sodium bicarbonate
Soda belongs to the chemical class of alkaline substances. Alkalis themselves are very useful because they can neutralize acids. Note that the level of acidity in the body is one of the main indicators of homeostasis, and its jump up or down can negatively affect human health. That is why soda powder is considered the mildest, natural way to combat abnormal acidity in the body. In turn, it is worth remembering that soda can have an aggressive effect on the gastric mucosa, so it must be taken with extreme caution. In order not to turn soda powder from an assistant into the culprit of further health problems, you do not need to ingest concentrated solutions of it. Moderation is important in everything.
When soda powder is dissolved in water, an alkali solution is obtained.
This is the main effect for which patients use it for acidosis. If you consume more than the recommended dose, alkalization of the blood may occur, which will have an extremely negative impact on human health.
Not everyone can recommend soda powder, since a healthy person with normal acid-base balance risks suffering from alkalosis, another negative condition for the body. With alkalosis, a person experiences the following symptoms: pale skin, headache, lack of appetite, thirst, impaired cerebral circulation, cardiac activity, seizures, allergic manifestations. If these symptoms occur, you should stop taking soda and take Diacarb to improve your condition.
Application on the farm
The substance is also irreplaceable in everyday life. It is an excellent cleaning agent. To restore their shine, chrome items and silverware are rubbed with dry soda, washed with soapy water, and then wiped dry with a soft cloth.
Sodium bicarbonate powder applied to a damp sponge will remove scratches and abrasions on vinyl flooring. Tile, kitchen stove, sink and plumbing fixtures can be cleaned of dirt by treating them with a thick mixture of baking soda and water. The same mixture helps get rid of the specific cat odor in places where there were “marks”.
To remove odors
The good hygroscopicity of sodium bicarbonate is the reason why it quickly absorbs odors, so it can be used to eliminate various odors. To get rid of unpleasant odors in the refrigerator, you need to pour dry powder into a glass and place it in the refrigerator door. By changing the contents of the glass as needed (once every 1-2 months), you can permanently get rid of the specific “refrigeration” smell.
If the smell of sour milk persists, the “smelling” containers should be cleaned with dry powder. Do the same with dishes that smell of fish.
If you pour a few tablespoons of powder into the drain hole, and after a few minutes turn on warm water, you can eliminate the unpleasant odor from the siphon under the sink.
Baking soda will also help deal with the unpleasant odor from the carpet. To do this, sprinkle the carpet with powder, leave it for 20-30 minutes, and then vacuum it thoroughly. However, this method is only suitable for non-fading carpets.
Using baking soda, you can also prevent the appearance of unpleasant odors, for example, from a washing machine or dishwasher when they are idle for a long time. When leaving home for a long time, you should rub the inside surface of the machines with dry bicarbonate and leave their doors ajar, and after returning, run them in the rinsing mode.
For clothing care
When machine washing, it is good to add baking soda to the washing powder. This will help get rid of the unpleasant odor in the washing machine, improve the quality of washing and the aroma of washed clothes. Clothes that smell bad can be washed in a machine by sprinkling them generously with baking soda.
A wet swimsuit will not become moldy and will not smell unpleasant if, after swimming in a pool or natural body of water, you put it in a bag with soda, and rinse it thoroughly and dry it at home.
Where is sodium bicarbonate used?
A substance such as baking soda is in great demand and is used in various areas of human life. Entire industries, such as food, medicine and pharmaceuticals, light and chemical industries, cannot do without it. It is also needed in non-ferrous metallurgy. It goes to chain stores for people to buy and use for household purposes.
In the food industry it is used as an additive E500, and is used in the production of confectionery products and drinks. In the chemical industry, polystyrene foam, dyes, fillers in fire extinguishers, and household chemical products are produced using soda. Light industry uses sodium bicarbonate for the production of dermantine, as well as sole rubber. In medicine - to provide assistance for various disorders and diseases. Metallurgy uses it for the precipitation of rare earth metals, and also for the flotation of ores.
There are a number of industries that simply need sodium bicarbonate for their full functioning.
Food industry
When baking gingerbread, they use the property of sodium bicarbonate to react chemically with acid, forming carbon dioxide. This ability of soda has been known for a very long time; it is used to give structure and porosity to cookies. To do this, take sodium bicarbonate and quench it with vinegar. The resulting mixture begins to react violently with each other. Extinguishing soda is a spectacular sight: a lot of foam is formed and a loud hissing sound. You should quickly add it to the dough and mix thoroughly.
Sodium bicarbonate is registered as a food additive. Its marking is E500.
It would seem, what’s wrong with this soda, but in fact, the structure of the dough becomes completely different. The baked goods are made “fluffy”, soft and beautiful. Baking soda is used in baking, when various confectionery products are made. It can also sometimes be used to make sparkling drinks such as mineral and carbonated waters, sparkling wines.
The food industry will continually use baking soda.
Medicine and pharmaceuticals
Recently it became known that such a familiar substance as soda is one of the components of human blood. A number of studies have also revealed that sodium bicarbonate has amazing properties: it perfectly restores the acid-base balance in the digestive system, is able to restore cellular metabolism, does not allow potassium to be washed out of the body’s cells, and helps tissues absorb oxygen. From the above it follows that soda is an important first aid remedy.
Therefore, sodium bicarbonate is used against heartburn, as it has an antacid effect. The property of baking soda allows you to get rid of the unpleasant sensations that arise due to increased acidity. With the help of drinking a soda solution, the symptoms go away. But in medicine there are other options for using sodium bicarbonate:
- For diarrhea, nausea and vomiting, a salt-soda solution helps replenish lost water reserves and restore fluid balance in the body.
- For the treatment of cardiovascular diseases (hypertension, edema and arrhythmia), a non-concentrated soda solution is also taken.
- As a thinner that removes mucus from the respiratory system, thereby relieving a person from coughing. It is used for inhalation for colds.
- As first aid for minor burns.
- To destroy fungal infections, eliminate foot fungus, treat thrush with douching, and do rinses for conjunctivitis.
- As an assistant in freeing a person from heavy metal salts.
- When brushing teeth for a whitening effect.
- As a remedy to relieve skin rashes and relieve itching. This also applies to insect bites.
- To achieve an anti-inflammatory effect.
- When taking a warm bath, when soda is added to the water, together with essential oils, the body relaxes, a person gets rid of fatigue and stress is relieved.
- Pharmaceuticals use sodium bicarbonate to produce anti-tuberculosis medications, antibiotics, and injection solutions.
Baking soda, acting as a medicine or an effective assistant, is beneficial. Like any drug, it has contraindications and dosage recommendations. Incorrect use may be harmful.
Light and chemical industries
The chemical industry uses soda for its needs. With its help, means of fighting fire are created. Baking soda is an ingredient in powder fire extinguishers. It works by releasing carbon dioxide, pushing oxygen away from the ignition point.
In production, machines, machines and surfaces are cleaned from relevant contaminants using ASO (abrasive blast cleaning) technology. NaHCO3 acts as a grinding material. This cleaning method using baking soda is a high-quality technology in which this substance acts gently.
The abrasive is moved using compressed air when the compressor operates. The advantage of using this technology over sandblasting is that soda is more gentle than sand and does not scratch surfaces.
Light industry also uses sodium bicarbonate for surface treatment when making rubber soles and other products. Baking soda is also used in the production of leatherette and textile products. It is useful because it is a good additive and also a degreaser. It is used in tanning leather and making products. Also in the textile workshop, sodium bicarbonate does an excellent job of bleaching fabric. With prolonged contact with baking soda, burns can form, and this is a negative effect of sodium bicarbonate.
The light and chemical industries also cannot do without sodium bicarbonate.
Use in cosmetology
Baking soda is an excellent cosmetic product. A scrub made from crushed oatmeal and dry sodium bicarbonate has a good cleansing and whitening effect. After such a scrub, the skin becomes soft, and its regular use gets rid of acne. To add shine to your hair after washing your hair, you need to treat it with a solution of soda and lemon juice.
For weight loss
Sodium bicarbonate is also used for weight loss. To lose up to 2 kg in one procedure, you can fill a bath with warm water and dissolve 0.5 kg of sea salt and 0.3 kg of regular baking soda in it. Anyone losing weight needs to immerse themselves in such a bath for 20 minutes. In this case, the water temperature should be about 40°C. A soda-salt solution relaxes muscles, relieves fatigue and nervous tension, cleanses lymphatic vessels, and reduces tissue swelling. After a bath, you don’t need to dry yourself: just put on a warm robe. It is better to do such water procedures before bed.
Precautionary measures
To avoid health problems, take precautions when using soda as a medicine:
- Do not exceed the dosage indicated in the prescription.
- Do not use baking soda in combination with antibiotics or other potent drugs.
- Do not make independent intravenous infusions of soda solutions.
- If allergic reactions occur or the condition worsens, stop taking it immediately and consult a doctor.
- In the presence of complicated or chronic diseases, the use of sodium bicarbonate is possible only after medical consultation.
Pharmacy soda is an effective drug that is successfully used in medicine. However, like any medicine, it requires caution and compliance with dosages. Do not try to adjust the treatment yourself - this can cause serious harm to your health.
In the video, Alexander Zakurdaev, a friend of Professor Neumyvakin, talks about medical soda.
Industrial use
Baking soda as a food additive E500 is used by the food industry in the production of bakery, flour, confectionery, sausage products, carbonated drinks, as well as for cleaning industrial equipment.
The chemical industry uses sodium bicarbonate in the production of dyes, reagents, household chemicals, and foams. Powder fire extinguishers are filled with bicarbonate.
In light industry, soda is used in tanning, for the production of artificial leather, silk and cotton fabrics.
What is soda?
Baking soda is a sodium salt that neutralizes acids. It is so unique and diverse in its manifestations that it has received several names and is successfully used in various fields of human activity. For example, every housewife is accustomed to the name baking soda, but chemists call it sodium bicarbonate. Depending on the chemical composition, soda powder can change its properties, from which it received several more names - soda ash, crystalline soda and others.
Sodium bicarbonate is found in sufficient quantities in nature, but humans need to extract it. Entire soda lakes are known - there are deposits of this valuable substance in Tanzania, California, and northern Russia. The Americans are richest in soda powder - their own deposits satisfy the needs of the country's population by forty percent.
As before, soda is extracted from minerals in soda lakes. Even in the first century AD, cases of obtaining soda powder from the evaporation of water from soda lakes became known, but the pure substance was obtained only in the first half of the 18th century, when one French naturalist de Monceau was able to obtain it experimentally. Since that time, factories for the production of soda powder, so necessary in the food, chemical and light industries, began to appear all over the world, including in Russia. And recently, sodium bicarbonate has deservedly occupied its niche in medicine.