Pharmacodynamics and pharmacokinetics
The medicinal properties of the drug are due to the presence of iron in its composition in combination with ascorbic acid .
Iron , in itself, is an integral part of the human body, its important functional unit. It is part of hemoglobin and takes part in oxidative reactions in tissues. Ascorbic acid improves the quality and affects the rate of absorption of iron by the body.
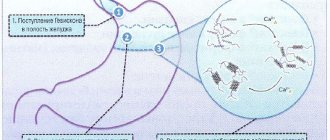
The special form, matrix , in which the drug is released, ensures a gradual and slow release of iron. The process does not take place in the stomach, but in the duodenum and jejunum , under the action of peristalsis . The process is characterized by a high degree of absorption and bioavailability . More than 90% of active iron is bound to blood proteins. The half-life is approximately six hours.
Contraindications
- drug allergy;
- hemosiderosis and hemochromatosis ;
- other types of anemia ;
- thrombosis and thrombophlebitis ;
- diabetes mellitus and fructose intolerance ;
- stenosis of the gastrointestinal tract;
- urolithiasis and other serious kidney diseases;
- children (up to 12 years old);
- parallel use of drugs containing iron, problems with the absorption of iron in the body.
For inflammation and peptic ulcers of the gastrointestinal tract, the drug is used only as directed and under the supervision of a physician.
Overdose
Symptoms:
abdominal pain, vomiting and diarrhea mixed with blood, fatigue or weakness, hyperthermia, paresthesia, pale skin, cold clammy sweat, acidosis, weak pulse, decreased blood pressure, palpitations. In case of severe overdose, signs of peripheral circulatory collapse, coagulopathy, hyperthermia, hypoglycemia, liver damage, renal failure, muscle cramps and coma may appear after 6-12 hours.
Treatment:
in case of overdose, consult a doctor immediately. It is necessary to rinse the stomach, inside a raw egg, milk (to bind iron ions in the gastrointestinal tract); deferoxamine is administered. Symptomatic therapy.
Side effects
The most common side effects are:
- constipation, diarrhea, nausea and pain in the epigastric region;
- redness and itching of the skin, possible Quincke's edema ;
- hyperglycemia;
- taking the drug promotes the formation of stones and sand in the kidneys;
- thrombocytosis, erythrocytopenia, leukocytosis;
- deviations from the normal content and metabolism of zinc and copper in the body are possible;
- headache, weakness, rapid heartbeat, dizziness;
- blackening or darkening of stool.
Instructions for use Sorbifer Durules (Method and dosage)
According to the instructions for Sorbifer, the tablet is swallowed whole, without chewing or breaking into parts, and washed down with water.
As a rule, unless your doctor prescribes otherwise, the daily dose is 2 tablets (no more than four), divided into two doses. The course of treatment can be 3-4 months.
How to take the drug during pregnancy?
For preventive purposes, one tablet per day is prescribed, during treatment - 2 tablets. Therapy continues until iron levels in the body are restored.
Sorbifer Durules No. 50 tab.p.o.
Instructions for medical use of the drug Sorbifer® Durules® Trade name Sorbifer® Durules® International nonproprietary name No Dosage form Film-coated tablets Composition One tablet contains active ingredients: iron (II) sulfate dry 320 mg (equivalent to 100 mg iron (II)) , ascorbic acid 60 mg, excipients: povidone (K-25), polyethene powder, carbomer 934 P, magnesium stearate, shell composition: hypromellose, macrogol 6000, titanium dioxide E 171, iron (III) yellow oxide E 172, solid paraffin . Description Tablets are lenticular-shaped, slightly biconvex, ocher-coated, yellow in color, engraved “Z” on one side, with a characteristic odor. Pharmacotherapeutic group Hematopoiesis stimulants. Iron supplement. Fe++ preparations for oral administration. ATC code B03A A Pharmacological properties Pharmacokinetics "Durules" is a special production technology that ensures the gradual release of the active substance (iron ions), a uniform supply of the drug. Iron is absorbed primarily in the duodenum and proximal jejunum. Taking 100 mg twice a day provides 30% greater absorption of iron from Sorbifer Durules compared to conventional iron supplements. Absorption and bioavailability of iron are high. Bonding with plasma proteins is 90% or more. Deposited in the form of ferritin and hemosiderin in hepatocytes and cells of the phagocytic macrophage system, a small amount is deposited in the form of myoglobin in muscles. The half-life is 6 hours. The presence of ascorbic acid in the preparation creates more favorable conditions for the absorption of iron in the intestines. At the molecular level, ascorbic acid mobilizes iron from the crystalline core of ferritin in vitro, reducing Fe 3+ to Fe 2+. At the intracellular level, ascorbic acid enhances iron-induced ferritin translation by promoting the conversion of iron regulatory protein (IRP) from its RNA-bound form to aconitase. Pharmacodynamics Iron is an essential component of the body, necessary for the formation of hemoglobin and the implementation of oxidative processes in living tissues. The active substance is contained in a biologically indifferent plastic matrix with a spongy structure. When passing through the gastrointestinal tract from the porous matrix of the tablets (within 6 hours), a continuous release of ferrous ions occurs. The film coating of the tablet and the porous matrix provide long-term release of iron ions. The film coating of the tablet, which completely disintegrates under the action of intestinal peristalsis and releases the active ingredient, helps prevent the tablet from dissolving in the stomach. The slow release of iron ions does not lead to the creation of a high local concentration, which avoids irritation of the mucous membrane of the gastrointestinal tract. Ascorbic acid slows down the breakdown of ferritin by blocking autophagy of ferritin by ferritin lysosomes and transformation into hemosiderin. Ascorbic acid accelerates the absorption of iron in the gastrointestinal tract, reducing unbound heme iron (III) to iron (II) in the stomach. Indications for use - iron deficiency anemia - latent iron deficiency in the body (without anemia), associated with excessive iron losses (bleeding, including uterine bleeding, constant donation), malnutrition - conditions accompanied by an increased need for iron in the body (prevention during pregnancy, lactation and in blood donors) Method of administration and dosage The tablet should be taken whole, without chewing, and at least half an hour before meals, with half a glass of water. Adults and adolescents over 12 years of age: Recommended dose: 1 tablet 2 times a day (morning and evening). For patients with grade II-III iron deficiency anemia, if necessary, on the recommendation of a doctor, the dose can be increased to 3-4 tablets 2 times a day (morning and evening) with a duration of use of 3-6 months. During pregnancy: The drug is used in case of established iron deficiency (with iron deficiency anemia and latent iron deficiency in the body) Prophylactic dose: 1 tablet per day. Therapeutic dose: 1 tablet 2 times a day (morning and evening). Treatment should be carried out until hemoglobin levels normalize and continue until the iron depot is completely saturated for another 2 months. Individual long-term therapy (with or without interruptions) is indicated for regularly occurring significant iron loss (for example, with heavy menstruation). Side effects The frequency of side effects from the digestive system increases with increasing doses from 100 to 400 mg per day. Often (>1/100) - nausea, abdominal pain, diarrhea, constipation Rarely (<1/100) - ulcerative lesions of the esophagus, esophageal stenosis - allergic reactions (itching, rash, hyperemia) - hyperthermia Contraindications - hypersensitivity to active or any other inactive component of the drug - esophageal stenosis and/or other obstructive changes in the digestive tract - increased iron content in the body (hemosiderosis, hemochromatosis) - repeated blood transfusions - impaired iron utilization (lead anemia, sideroblastic anemia, hemolytic anemia) - children under 12 years of age (due to lack of clinical data) Drug interactions Concomitant use of Sorbifer Durules with the following drugs is not recommended: - ciprofloxacin: simultaneous use reduces the absorption of ciprofloxacin by approximately 50%, so there is a risk that the content of ciprofloxacin in the blood plasma will be lower than necessary for therapeutic action. - levofloxacin: simultaneous use reduces the absorption of levofloxacin. -moxifloxacin: simultaneous use reduces the bioavailability of moxifloxacin by almost 40%, therefore, if simultaneous use is required, it is necessary to ensure the longest possible period between taking moxifloxacin and Sorbifer Durules. - norfloxacin: simultaneous use reduces the absorption of norfloxacin by approximately 75%. - ofloxacin: simultaneous use reduces the absorption of ofloxacin by approximately 30%. When using Sorbifer Durules concomitantly with the following drugs, dosage adjustment of these drugs may be required. The recommended minimum time interval between taking Sorbifer Durules and these drugs should be at least 2 hours: - calcium or dietary supplements containing magnesium carbonate, as well as aluminum hydroxide or calcium, antacids containing magnesium carbonate, together with iron salts form a complex that reduces absorption each other. - Captopril: Concomitant use reduces the area under the plasma concentration-time curve for captopril by approximately 37%, presumably due to chemical reactions in the gastrointestinal tract. - zinc: simultaneous use reduces the absorption of zinc salts. - clodronate: simultaneous use may reduce the absorption of clodronate. - deferoxamine: with simultaneous use, the absorption of deferoxamine and iron is reduced due to the formation of a compound. - levodopa: when used concomitantly, ferrous sulfate reduces the bioavailability of single doses of levodopa by approximately 50%, and of single doses of carbidopa by 75%, possibly due to the formation of a chelate. - methyldopa: with simultaneous use, the bioavailability of methyldopa is reduced, possibly due to the formation of a chelate. - penicillamine: simultaneous use of penicillamine and iron salts reduces their absorption, possibly due to the formation of a chelate compound. - risedronate: in vitro studies have shown that preparations containing iron form compounds with risedronate. Although in vivo drug interaction studies have not been conducted, concomitant use of these drugs may reduce the absorption of risedronate. - tetracyclines: with simultaneous use, the absorption of tetracyclines and iron is reduced. If simultaneous use is necessary, the recommended minimum time interval between taking Sorbifer Durules and these drugs should be at least 3 hours. When taken orally, iron inhibits the enterohepatic circulation of oxytetracyxine (doxycycline), as well as when administered intravenously. - thyroid hormones: with the simultaneous use of iron and thyroxine preparations, the absorption of thyroxine may be reduced, which reduces the effectiveness of replacement therapy. -cimetidine: with simultaneous use, the decrease in gastric acid production caused by cimetidine reduces the absorption of iron. Therefore, the intervals between taking these drugs should be at least 2 hours. -chloramphenicol: when taken simultaneously, the clinical effect of iron treatment may be delayed. When taking the drug simultaneously with tea, coffee, eggs, dairy products, wheat bread, porridge or foods rich in plant fiber, iron absorption may be reduced. Special instructions Iron supplements can cause poisoning in children. When using the drug, darkening of the stool appears, which has no clinical significance. The drug is used with caution for gastric and duodenal ulcers, inflammatory bowel diseases (enteritis, diverticulitis, ulcerative colitis, Crohn's disease), chronic liver and kidney diseases. During the treatment period, it is recommended to monitor the concentration of hemoglobin and iron in the blood. Comprehensive laboratory and instrumental monitoring of the effectiveness of treatment is recommended to be carried out every 7-14 days, depending on the course of anemia. Pregnancy and lactation The drug can be used during pregnancy and lactation. Features of the effect of the drug on the ability to drive a vehicle or potentially dangerous mechanisms. Does not affect. Overdose Symptoms: abdominal pain, vomiting and diarrhea (sometimes with blood), fatigue, weakness, hyperthermia, paresthesia, pale skin, cold clammy sweat, acidosis, weak pulse, decreased blood pressure, palpitations. Signs of peripheral circulatory collapse, coagulopathy, hyperthermia, hypoglycemia, liver damage, renal failure, muscle cramps and coma may appear after 6-12 hours. Treatment: gastric lavage, milk and raw eggs orally, drugs that provoke vomiting, symptomatic therapy. If necessary, perform a gastric lavage with a solution of deferoxamine at a concentration of 2 g/l, then 5 g of deferoxamine is dissolved in 50-100 ml of water and this solution is left in the stomach. In case of severe intoxication: in a state of shock and/or coma and in case of increased serum iron levels (> 90 mmol/l in children, > 142 mmol/l in adults), intensive care should be started immediately and deferoxamine should be administered (15 mg/l). kg/h intravenously slowly, maximum 80 mg/kg/24 hours). Too high an infusion rate may result in hypotension. In less severe cases of intoxication, deferoxamine can be administered intramuscularly (50 mg/kg, total dose not more than 4 g). It is recommended to monitor serum iron throughout the course of treatment. Release form and packaging 30 and 50 tablets are placed in brown glass bottles, sealed with a polyethylene cap and equipped with an accordion shock absorber for glass bottles. The bottle, together with instructions for medical use in the state and Russian languages, is placed in a lithographed cardboard box. Storage conditions Store at a temperature not exceeding 25 °C. Keep out of the reach of children! Shelf life 3 years Do not use after expiration date. Conditions for dispensing from pharmacies Without a prescription 1106 BUDAPEST, st. Keresturi, 30-38 HUNGARY Tel.: (36-1) 803-5555, Fax: (36-1) 803-5529 Under license from AstraZeneca, Sweden Registration certificate holder JSC "EGIS Pharmaceutical Plant", Hungary Address of the organization receiving the territory of the Republic of Kazakhstan, claims from consumers regarding the quality of products (products) Representative office in the Republic of Kazakhstan CJSC "EGIS Pharmaceutical Plant" 050060, Almaty, st. Zharokova 286 G tel: +, +, fax: + 7 (727) 247 61 41, e-mail
Interaction
You should not combine the drug with ciprofloxacin, norfloxacin, levofloxacin, ofloxacin and moxifloxacin .
The combination of dietary supplements containing calcium and magnesium, captopril, clodronate, cimetidine, levodopa, zinc, desferoxamine, methyldopa, tetracycline, penicillinamine, thyroid hormones, pancreatin, ethanol and tocopherol with Sorbifer is not recommended. The interval between taking medications should be less than two hours.
Combining the drug with ascorbic acid can lead to an overdose of iron.
The absorption process of the drug worsens when combined with milk, eggs, tea, coffee, juices, bread, and foods rich in plant fiber. Oral contraceptives and chloramphenicol also negatively affect absorption .
Analogues of Sorbifer Durules
Level 4 ATC code matches:
Ferrogradumet
Hemofer
Tardiferon
Feroplekt, hemoferon, feron forte, globigen, actiferin, gemsinerad-td, totema, ranferol-12.
The price of analogues is slightly lower than that of the original.
Which is better: Maltofer or Sorbifer?
The drugs are almost identical; there is an opinion that maltofer is better absorbed and has fewer side effects. Everything is individual.
Which is better: Sorbifer or Fenyuls?
Fenyuls contains half as much iron as Sorbifer. Helps with minor anemia. The original copes with the symptoms of the disease faster.
Sorbifer during pregnancy and lactation
Sorbifer during pregnancy is prescribed for anemia .
According to the instructions, in the initial stages of pregnancy (the first six months), it is recommended to take one tablet of the drug once a day. Last trimester and when breastfeeding - 1 tablet twice a day.
Treatment is continued until the level of iron in the blood normalizes. As prescribed by the doctor, the medication can be extended up to 6 months.
Dosage regimen
I take the drug orally. Film-coated tablets should not be split or chewed. The tablet should be swallowed whole and washed down with at least half a glass of liquid.
Adults and teenagers
1 tablet is prescribed. 1-2 times/day. If necessary, for patients with iron deficiency anemia, the dose can be increased to 3-4 tablets/day in 2 doses (morning and evening) for 3-4 months (until the iron depot in the body is replenished).
During pregnancy and lactation for the purpose of prevention
prescribed 1 tablet/day;
For treatment,
1 tablet is prescribed. 2 times/day (morning and evening).
Treatment should be continued until the optimal hemoglobin level is achieved. To further replenish the depot, you may need to continue taking the drug for another 2 months.
Price Sorbifer Durules (where to buy)
How much does it cost to buy the drug in Ukraine? On average, the price of 30 tablets is about 70 UAH. The cost of 50 tablets is about 80 UAH.
- Online pharmacies in RussiaRussia
- Online pharmacies in UkraineUkraine
- Online pharmacies in KazakhstanKazakhstan
ZdravCity
- Sorbifer Durules tablets p.p.o.
30 pcs. CJSC Pharmaceutical Plant EGIS RUR 394 order - Sorbifer Durules tablets p.p.o. 50 pcs. ZAO Farm.zavod EGIS
512 rub. order
Pharmacy Dialogue
- Sorbifer durules (tablet p/o 100mg+60mg No. 50)Egis
560 rub. order
- Sorbifer durules (tablet p/o 100mg+60mg No. 30)Egis
RUB 404 order
show more
Pharmacy24
- Sorbifer Durules No. 30 tablets ZAT FZ Egis, Ugorshchina
81 UAH. order - Sorbifer Durules No. 50 tablets ZAT FZ Yegis, Ugorshchina
107 UAH order
PaniPharmacy
- Sorbifer durules tablets Sorbifer durules tablets. p/o No. 30 Hungary, Egis
92 UAH order
- Sorbifer durules tablets Sorbifer durules tablets. p/o No. 50 Hungary, Egis
112 UAH order
show more
pharmachologic effect
Antianemic drug. Iron is an essential component of the body, necessary for the formation of hemoglobin and the occurrence of oxidative processes in living tissues.
Durules technology provides a gradual release of the active ingredient (iron ions) over a long period of time. The plastic matrix of Sorbifer Durules tablets is completely inert in the digestive juice, but completely disintegrates under the action of intestinal peristalsis, when the active ingredient is completely released.
Ascorbic acid helps improve iron absorption.