Pharmacodynamics and pharmacokinetics
Pharmacodynamics
The drug is for local use and has anti-allergic and anti-inflammatory effects. Enhances the production of phospholipase A2 ( lipocortin ), inhibits the process of release of arachidonic acid and the synthesis of its metabolic products. Reduces the exudation of the inflammatory process, reduces the intensity of granulation and infiltration, inhibits the migration of macrophages and the formation of chemotaxis, prevents the release of inflammatory mediators from cells, which plays an important role in the mechanism of symptoms of allergic chronic rhinitis .
The drug does not have MCS activity, is well tolerated by patients with long-term use, and does not have a resorptive effect.
Pharmacokinetics
Budesonide is quickly adsorbed after inhalation; the systemic bioavailability of the drug after inhalation through a nebulizer is about 15% of the administered dose. In the blood, the maximum concentration is reached on average after 30 minutes. Binding to blood proteins is high.
Biotransformation of budesonide occurs with the participation of the liver isoenzyme CYP3A4 . The main metabolites have extremely weak pharmacological activity. The drug is excreted by the kidneys, mainly in the form of metabolites (up to 70%) and in small quantities (about 10%) through the intestines.
Side effects
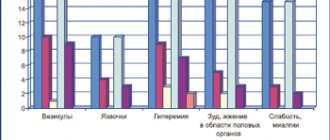
They manifest themselves mainly in the form of local reactions on the nasal mucosa (dryness, burning, irritation). Runny nose , atrophy or ulceration of the nasal mucosa, nosebleeds, sore throat, sneezing, sore throat, headache , allergic reactions on the skin, perforation of the nasal septum, myalgia , conjunctival hyperemia , nasal congestion, drowsiness , cough, nausea, vomiting, palpitations , candidiasis of the nasal and pharyngeal mucosa, growth retardation.
Budenit Steri-Neb, instructions for use (Method and dosage)
The drug is administered by inhalation through an inhaler-nebulizer . Before using it, you must carefully study the instructions and techniques for working with it.
The recommended daily dosage of the drug for severe bronchial asthma for inhaled GCS therapy, for adults and children over 12 years of age is 1–2 mg twice a day, a maintenance dose is 0.5–4 mg per day. At the age of 6 months to 12 years, the daily dosage ranges from 0.25 to 0.5 mg twice a day, the maintenance dose is 0.25–2 mg per day. If the prescribed dose is no more than 1 mg/day, then it can be taken in one dose.
As a rule, the maintenance dose is selected by the doctor individually, and when a therapeutic effect is achieved, it should be reduced to the lowest effective dose. If it is necessary to enhance the therapeutic effect, the dose of the drug can be increased or a combination of the drug with oral corticosteroids is recommended.
Budenit Steri-Neb suspension for inhalation 0.25 mg/ml 2 ml 20 pcs
Registration Certificate Holder
NORTON HEALTHCARE (UK)
Dosage form
Medicine - Budenit Steri-Neb
Description
Dosed suspension for inhalation
almost white, finely dispersed, practically odorless.
1 ml
budesonide 250 mcg
Excipients
: polysorbate 80 - 0.2 mg, sodium chloride - 8.5 mg, sodium citrate dihydrate - 0.5 mg, citric acid monohydrate - 0.31 mg, disodium edetate - 0.1 mg, water for injection - up to 1 ml.
2 ml - polyethylene ampoules, soldered together in the form of a block (5) - laminated foil (4) - cardboard packs with first opening control. 2 ml - polyethylene ampoules, soldered together in the form of a block (5) - laminated foil (12) - cardboard packs with first opening control.
Indications
- treatment of bronchial asthma (as a basic therapy; with insufficient effectiveness of beta2-adrenergic agonists; to reduce the dose of oral corticosteroids) in case of ineffectiveness or impossibility of using budesonide in an inhaler that pumps the drug into the respiratory tract, or an inhaler containing the drug in powder form;
- treatment of chronic obstructive pulmonary disease (COPD);
- stenosing laryngotracheitis (false croup).
Contraindications for use
- age up to 6 months;
- hypersensitivity to budesonide or any other component of the drug.
Carefully:
pulmonary tuberculosis; fungal, bacterial, parasitic and viral infections of the respiratory system; cirrhosis of the liver; pregnancy; lactation period.
pharmachologic effect
GCS with a pronounced local anti-inflammatory and antiallergic effect. Budesonide increases the production of lipocortin, which is an inhibitor of phospholipase A2, inhibits the release of arachidonic acid, inhibits the synthesis of leukotrienes and prostaglandins, reduces inflammatory exudation and the production of cytokines, inhibits the migration of macrophages, reduces the severity of infiltration and granulation processes, the formation of a chemotaxis substance (which explains the effectiveness in delayed hypersensitivity reactions type), inhibits the release of inflammatory mediators from mast cells (immediate hypersensitivity reaction).
Budesonide restores the patient's sensitivity to bronchodilators, allowing them to reduce the frequency of their use, reduces swelling of the bronchial mucosa, mucus production, sputum formation and reduces airway hyperresponsiveness. Increases mucociliary transport. It is well tolerated during long-term treatment and does not have mineralocorticoid activity.
The time for the onset of the therapeutic effect after inhalation of one dose of the drug is several hours. The maximum therapeutic effect is achieved 1-2 weeks after treatment. Budesonide effectively prevents attacks of bronchial asthma of physical exertion, but does not stop an acute attack of bronchospasm.
Drug interactions
The metabolism of budesonide is mainly carried out with the participation of the CYP3A4 isoenzyme. Taking ketoconazole at a dose of 100 mg 2 times a day increases the plasma concentration of orally taken budesonide at a dose of 10 mg once by an average of 7.8 times. There is no information on such interactions with inhaled dosage forms of budesonide, but a marked increase in plasma concentrations of the drug should be expected, therefore, CYP3A4 inhibitors such as ketoconazole and itraconazole may increase the systemic exposure of budesonide. Other strong CYP3A4 inhibitors are also likely to markedly increase budesonide plasma concentrations.
Pre-inhalation of beta-agonists dilates the bronchi, improves the entry of budesonide into the respiratory tract and enhances its therapeutic effect.
Phenobarbital, phenytoin, rifampicin reduce effectiveness (induction of microsomal liver enzymes).
Methandienone and estrogens enhance the effect of budesonide.
Pharmaceutical interactions
The drug Budenit Steri-Neb can be mixed with 0.9% sodium chloride solution and with other solutions intended for use with nebulizers, for example, with terbutaline, salbutamol, fenoterol, acetylcysteine, sodium cromoglycate or ipratropium bromide.
Dosage regimen
Budenit Steri-Neb is used by inhalation using nebulizer inhalers (see below - “Technique of use”).
Recommended doses of the drug in case of initiation of inhaled GCS therapy for severe bronchial asthma, as well as against the background of dose reduction or discontinuation of oral GCS
for
adults (including the elderly) and children over 12 years old
- 1-2 mg 2 times / day, maintenance dose - 0.5-4 mg / day;
for children from 6 months to 12 years
- 0.25-0.5 mg 2 times / day, maintenance dose - 0.25-2 mg / day. If the recommended dose does not exceed 1 mg/day, the entire dose of the drug can be taken at one time (at one time).
The maintenance dose must be selected individually. When a therapeutic effect is achieved, the maintenance dose must be reduced to the lowest dose at which the patient has no symptoms of the disease: for adults (including the elderly) and children over 12 years of age
— 0.5-1 mg 2 times/day;
for children from 6 months to 12 years
- 0.25-0.5 mg 2 times / day.
Dose conversion table for patients receiving oral corticosteroids in terms of budesonide
Dose (mg) of budesonide taken orally Budenit Steri-Nab 0.5 mg/2 ml (0.25 mg/ml) volume (ml) Budenit Steri-Nab 1 mg/2 ml (0.5 mg/ml) volume (ml)
0.251-0.5210.753-1421.563284
If it is necessary to achieve an additional therapeutic effect, it can be recommended to increase the dose of Budenit Steri-Neb instead of combination with oral corticosteroids (to reduce the risk of developing systemic effects).
For stenosing laryngotracheitis (false croup)
in children aged 6 months and older,
the recommended dose is 2 mg/day at a time or in 2 doses of 1 mg at intervals of 30 minutes.
Technique of use
Ultrasonic nebulizers are not suitable for use with Budenit Steri-Neb. The dose required by the patient may vary depending on the nebulizer used. Inhalation time and dose of the drug depend on the air flow speed, the volume of the nebulizer chamber and the filling volume. Therefore, to inhale the drug Budenit Steri-Neb, it is necessary to use an appropriate nebulizer, as well as a mouthpiece and a special face mask. The nebulizer must be connected to an air compressor to create adequate air flow. Before using the drug, you must read the instructions from the nebulizer manufacturer.
1. Prepare the nebulizer according to its manufacturer's instructions.
2. Separate Steri-Neb (the ampoule with a sterile solution) from the block by turning and pulling it.
3. Hold the ampoule vertically with the cap up and break off the cap.
4. Squeeze the solution into the nebulizer reservoir.
5. Use the nebulizer according to its manufacturer's instructions.
You should rinse your mouth after finishing inhalation. If you used a mask, you must rinse your face.
Any solution remaining unused in the nebulizer chamber should be discarded. Wash the nebulizer thoroughly.
When using the drug, you should avoid getting the solution into your eyes.
Overdose
In case of acute overdose
Budesonide usually does not cause clinical manifestations.
Treatment:
drug withdrawal, inhalation of short-acting bronchodilators.
With long-term use in doses higher than recommended,
A systemic glucocorticoid effect may develop in the form of hypercortisolism and suppression of adrenal function.
Side effect
Often (≥1/100, <1/10):
irritation and dryness of the pharyngeal mucosa, candidal stomatitis, hoarseness, cough, dryness of the oral mucosa, unpleasant taste.
Rarely (≥1/10,000, <1/1000):
nervousness, excitability, depression, behavioral disturbances, immediate and delayed hypersensitivity reactions (including rash, contact dermatitis, urticaria, angioedema and bronchospasm), skin bruising or thinning of the skin, headache, nausea, esophageal candidiasis.
Systemic effects may occur during inhaled treatment with GCS.
, especially with long-term treatment with high doses. The likelihood of such effects occurring is significantly less than with the treatment of oral corticosteroids. Possible systemic effects include adrenal suppression, growth retardation in children and adolescents, decreased bone mineral density, cataracts, and glaucoma.
The drug Budenit Steri-Neb contains 0.1 mg/ml disodium edetate, which can cause bronchospasm at concentrations above 1.2 mg/ml.
As with other inhaled therapies, paradoxical bronchospasm may occur with rapid worsening of dyspnea after dosing. If a severe reaction occurs, alternative therapy should be considered.
In some cases, facial skin irritation occurs when using a nebulizer with a mask. To prevent irritation, after using the mask, the facial skin should be rinsed with water.
special instructions
The drug Budenit Steri-Neb is not intended for rapid relief of attacks of bronchial asthma; to relieve acute bronchospasm, it is recommended to use short-acting inhaled bronchodilators.
Patients not receiving GCS
Usually the therapeutic effect occurs within 10 days. In patients with excessive mucus secretion in the bronchi, a short (about 2 weeks) additional treatment with oral corticosteroids may initially be given. After a course of oral therapy, in many cases it is possible to completely stop taking GCS orally.
Patients on GCS therapy
Before transferring a patient from treatment with oral corticosteroids to treatment with Budenit Steri-Nab, the patient's condition should be relatively stable. After which the drug Budenit Steri-Neb is used in combination with the previously used dose of GCS for oral administration for about 10 days. Subsequently, the dose of oral corticosteroids should be gradually reduced (for example, by 2.5 mg of prednisolone or its equivalent every month) as far as possible to the lowest level. In most cases, oral GCS can be completely replaced with Budenit Steri-Neb.
Sometimes, during a transfer from treatment with oral corticosteroids to treatment with Budenit Steri-Neb, symptoms that were previously relieved by systemic drugs are observed: for example, rhinitis, eczema, muscle and joint pain. The occurrence of symptoms such as fatigue, headache, nausea and vomiting may indicate the development of systemic insufficiency of GCS. In such cases, it may even be necessary to temporarily increase the dose of oral corticosteroids.
Systemic side effects of inhaled corticosteroids can occur primarily when high doses are administered over a long period of time. The likelihood of this effect occurring is significantly less than with treatment with oral corticosteroids. Possible systemic effects include adrenal suppression, growth retardation in children and adolescents, decreased bone mineral density, cataracts, and glaucoma. Therefore, it is very important to titrate the dose of inhaled corticosteroids to the lowest dose that maintains effective disease control. It is recommended to regularly monitor growth in children receiving inhaled corticosteroids for an extended period of time. In case of growth retardation, treatment should be adjusted to reduce the dose of GCS for inhalation to the lowest dose at which effective control of bronchial asthma is maintained.
Oral administration of ketoconazole and itraconazole
or other inhibitors of the CYP3A4 isoenzyme
increases the systemic exposure of budesonide. Therefore, if combined use is necessary, they should be taken at maximum intervals. A dose reduction of budesonide should also be considered.
To minimize the risk of fungal stomatitis, the patient and/or the child’s parents should be informed about the need to rinse the mouth with water after each inhalation of the drug.
Effect on the ability to drive vehicles and operate machinery
The drug Budenit Steri-Neb does not have a negative effect on the ability to drive a vehicle and operate machinery. In case of rare adverse reactions from the nervous system, activities that require rapid psychomotor reactions should be avoided.
Storage conditions
The drug should be stored out of the reach of children at a temperature not exceeding 25°C.
Best before date
Shelf life: 2 years. Do not use after the expiration date.
Use during pregnancy and breastfeeding
Restrictions during pregnancy - With caution. Restrictions when breastfeeding - With caution.
The use of budesonide during pregnancy is possible only if the benefit to the mother outweighs the possible risk to the fetus. If necessary, use the drug in the minimum effective dose.
There are no data on the excretion of budesonide in breast milk. Prescribing the drug during lactation is possible only under the supervision of a physician in cases where the expected benefit to the mother outweighs the possible risk to the child.
Use for liver dysfunction
Restrictions for liver dysfunction - With caution.
The drug should be prescribed with caution for liver cirrhosis.
Use in elderly patients
Restrictions for elderly patients - No restrictions.
Use in children
Restrictions for children - With caution. Use in children under 6 months is contraindicated.
Terms of sale
The drug is available with a prescription.
Contacts for inquiries
TEVA (Israel)
Teva LLC
115054 Moscow Valovaya st. 35 Business Tel.
Analogs of Budenit Steri-Neb
Level 4 ATX code matches:
Alvesco
Asmanex
Beclazon
Budesonide
Flixotide
Pulmicort
Aldecin
Apulein , Budecort , Gorakort , Benacort , Budesonide , Benarin Budenofalk , Buderin , Cicortide Cyclocaps , Budiair , Pulmicort , Tafen Nasal .
Reviews of Budenit Steri-Neb
Reviews of the drug from most patients are positive:
- “... The doctor diagnosed my child with chronic obstructive bronchitis, bronchial asthma is in question. We periodically undergo treatment in the allergy department”;
- “... After discharge, the doctor prescribed Budenit Steri-Neb inhalations for severe wheezing and coughing attacks. The drug stops attacks well, but we are afraid to take it often, because it contains hormones.”
Many patients on forums on the Internet are interested in: “Budenit Steri-Neb or Pulmicort - which is better?” If we talk about an inhalation suspension as a form of release of the drug for a nebulizer, then there are no significant differences. Same active ingredient, similar indications for use. However, there is a slight difference in the cost of these drugs in the pharmacy chain.
BUDENIT STERI-NEB 0.25MG/ML 2ML N20 SUSP D/ING DOZER
The drug Budenit Steri-Neb is not intended for rapid relief of attacks of bronchial asthma. To relieve acute bronchospasm, it is recommended to use short-acting inhaled bronchodilators.
Patients not receiving GCS
Usually the therapeutic effect occurs within 10 days. In patients with excessive mucus secretion in the bronchi, short (about 2 weeks) additional treatment with oral corticosteroids may initially be given. After a course of oral therapy, in many cases it is possible to completely stop taking GCS orally.
Patients on GCS therapy
Before transferring a patient from treatment with oral corticosteroids to treatment with Budenit Steri-Nab, the patient's condition should be relatively stable. After which the drug Budenit Steri-Neb is used in combination with the previously used dose of GCS for oral administration for about 10 days. Subsequently, the dose of oral corticosteroids should be gradually reduced (for example, by 2.5 mg of prednisolone or its equivalent every month) as far as possible to the lowest level. In most cases, oral GCS can be completely replaced with Budenit Steri-Neb.
Sometimes, during the transfer from treatment with oral corticosteroids to treatment with Budenit Steri-Neb, symptoms that were previously relieved by systemic drugs are observed: for example, rhinitis, eczema and muscle and joint pain. The occurrence of symptoms such as fatigue, headache, nausea and vomiting may indicate the development of systemic insufficiency of GCS. In such cases, it may even be necessary to temporarily increase the dose of oral corticosteroids.
Systemic side effects of inhaled corticosteroids may occur primarily when high doses are administered over a long period of time. The likelihood of this effect occurring is significantly less than with treatment with oral corticosteroids. Possible systemic effects include adrenal suppression, growth retardation in children and adolescents, decreased bone mineral density, cataracts, and glaucoma. Therefore, it is very important to titrate the dose of inhaled corticosteroids to the lowest dose at which effective disease control is maintained. It is recommended to regularly monitor growth in children receiving inhaled corticosteroids for an extended period of time. In case of growth retardation, treatment should be adjusted to reduce the dose of GCS for inhalation to the lowest dose at which effective control of bronchial asthma is maintained.
Oral administration of ketoconazole and itraconazole or other inhibitors of the CYP3A4 isoenzyme increases the systemic exposure of budesonide. Therefore, if combined use is necessary, they should be taken at maximum intervals. A dose reduction of budesonide should also be considered.
To minimize the risk of fungal stomatitis, the patient and/or the child’s parents should be informed about the need to rinse the mouth with water after each inhalation of the drug.
Impact on the ability to drive a vehicle and operate machinery.
The drug Budenit Steri-Neb does not have a negative effect on the ability to drive a vehicle or operate machinery. In case of rare adverse reactions from the nervous system, activities that require rapid psychomotor reactions should be avoided.
Technique of use
Ultrasonic nebulizers are not suitable for use with Budenit Steri-Neb. The dose required by the patient may vary depending on the nebulizer used. Inhalation time and dose of the drug depend on the air flow speed, the volume of the nebulizer chamber and the filling volume. Therefore, to inhale the drug Budenit Steri-Neb, it is necessary to use an appropriate nebulizer, as well as a mouthpiece and a special face mask. The nebulizer must be connected to an air compressor to create adequate air flow.
Before using the drug, you must read the instructions from the nebulizer manufacturer.
1. Prepare the nebulizer according to its manufacturer's instructions.
2. Separate STERI-NEB (ampoule with a sterile solution) from the block by turning and pulling it (Fig. 1).
Picture 1.
3. Holding the ampoule vertically with the cap up, break off the cap (Fig. 2).
Figure 2.
4. Squeeze the solution into the nebulizer reservoir (Fig. 3).
Figure 3.
5. Use the nebulizer according to its manufacturer's instructions.
6. Rinse your mouth after finishing inhalation.
7. If a mask was used, it is necessary to rinse your facial skin.
8. The solution remaining unused in the nebulizer chamber should be discarded.
9. Wash the nebulizer thoroughly.
10. When using the drug, avoid getting the solution into the eyes.
Price Budenit Steri-Neb, where to buy
The price of Budenit Steri-Neb suspension ampoules 0.25 mg/ml, 2 ml, No. 20 varies from 725 to 1120 rubles per package. Ampoules 0.5 mg/ml 2 ml No. 20 988 - 1280 rubles. You can buy Budenit Steri-Neb in the pharmacy chain of Moscow and other Russian cities without any difficulty.
- Online pharmacies in RussiaRussia
ZdravCity
- Budenit Steri-Neb susp.
d/ing. dosed 0.25 mg/ml 2 ml 60 pcs Norton Healthcare Limited RUB 1,582 order - Budenit Steri-Neb susp. d/ing. dosed 0.25 mg/ml amp. 2ml 20pcs Ivax Pharmaceuticals Ukey Limited
RUR 545 order
Compound
Active substance: budesonide
Excipients:
- polysorbate 80 - 0.2 mg;
- sodium chloride - 8.5 mg;
- sodium citrate dihydrate - 0.5 mg;
- citric acid monohydrate - 0.31 mg;
- disodium edetate - 0.1 mg;
- water for injection - up to 1 ml.
pharmachologic effect
Pharmacological action - antiexudative, antiallergic, anti-inflammatory.
Directions for use and doses
Inhalation using inhalers-nebulizers (see Techniques of use).
Recommended doses of the drug in case of initiation of inhaled GCS therapy for severe bronchial asthma, as well as against the background of dose reduction or discontinuation of oral GCS, are as follows:
Adults (including the elderly) and children over 12 years of age: usually 1–2 mg 2 times daily. The maintenance dose is 0.5–4 mg/day.
Children from 6 months to 12 years: 0.25–0.5 mg 2 times a day. The maintenance dose is 0.25–2 mg/day. If the recommended dose does not exceed 1 mg/day, the entire dose of the drug can be taken at one time (at one time).
The maintenance dose must be selected individually. When a therapeutic effect is achieved, the maintenance dose should be reduced to the lowest effective dose.
Adults (including the elderly) and children over 12 years of age: 0.5–1 mg 2 times a day.
Children from 6 months to 12 years: 0.25–0.5 mg 2 times a day.
If it is necessary to achieve an additional therapeutic effect, it can be recommended to increase the dose of Budenit Steri-Neb instead of combination with oral corticosteroids (to reduce the risk of developing systemic effects).
Below is a dose conversion table for patients receiving oral corticosteroids in terms of budesonide.
Table
| Dose of budesonide taken orally, mg | Volume of the drug Budenit Steri-Neb, 0.5 mg/2 ml (0.25 mg/ml), ml | Volume of the drug Budenit Steri-Neb, 1 mg/2 ml (0.5 mg/ml), ml |
| 0,25 | 1 | — |
| 0,5 | 2 | 1 |
| 0,75 | 3 | — |
| 1 | 4 | 2 |
| 1,5 | 6 | 3 |
| 2 | 8 | 4 |
Stenosing laryngotracheitis (false croup). Children from 6 months and older: the recommended dose is 2 mg/day at a time or in 2 divided doses, 1 mg at intervals of 30 minutes.
Technique of use
Ultrasonic nebulizers are not suitable for use with the drug Budenit Steri-Neb; the dose required by the patient may vary depending on the nebulizer used. Inhalation time and dose of the drug depend on the air flow speed, the volume of the nebulizer chamber and the filling volume. Therefore, to inhale the drug Budenit Steri-Neb, it is necessary to use an appropriate nebulizer, as well as a mouthpiece and a special face mask. The nebulizer must be connected to an air compressor to create adequate air flow.
Before using the drug, you must read the instructions from the nebulizer manufacturer.
1. Prepare the nebulizer according to its manufacturer's instructions.
2. Separate the ampoule with the sterile solution from the block by turning and pulling it (Fig. 1).
3. Holding the ampoule vertically with the cap up, break off the cap (Fig. 2).
4. Squeeze the solution into the nebulizer reservoir (Fig. 3).
5. Use the nebulizer according to its manufacturer's instructions.
6. Rinse your mouth after finishing inhalation.
7. If a mask was used, it is necessary to rinse your facial skin.
8. The solution remaining unused in the nebulizer chamber should be discarded.
9. Wash the nebulizer thoroughly.
10. When using the drug, avoid getting the solution into the eyes.
Release form
Suspension for inhalation dosed, 0.25 mg/ml and 0.5 mg/ml. In LDPE ampoules, 2 ml. 5 amp. soldered to each other in the form of a block. 1 block in laminated foil. 4 or 12 blocks in a cardboard box.
Manufacturer
Ivax Pharmaceuticals UK Limited, Preston Brook, Runcorn, Cheshire WA7 3FA, United Kingdom.
Marketing Authorization Holder: Norton Healthcare Limited, trading as Ivax Pharmaceuticals UK, United Kingdom.
Address for receiving claims: 119049, Moscow, st. Shabolovka, 10, building 2, Business.
Tel/fax/35/36.
Conditions for dispensing from pharmacies
- On prescription.
- OXYGEN-BUD-INFblock-040914-MEDIA-647-030915
Storage conditions for the drug Budenit Steri-Neb
- At a temperature not exceeding 25 °C.
- Keep out of the reach of children.
Shelf life of the drug Budenit Steri-Neb
- 2 years.
- Do not use after the expiration date stated on the package.