A complex drug based on calcium salt helps restore metabolism and prevent the development of many pathologies. Affordable and effective, it restores the normal level of one of the main macroelements in the body, relieving a variety of painful symptoms.
Composition and dosage form
Calcium chloride (CaCl2) is formed during the production of baking soda. The substance is used not only in medicine, but also in cooking, cosmetology, and the chemical industry. Without it, it is impossible to produce healthy calcined cottage cheese and hard cheeses.
The pharmaceutical form of calcium chloride is a water-based injection solution. Available in transparent glass ampoules of 5 or 10 ml. The cardboard packaging of the drug contains 10 such ampoules. The concentration of the medicinal compound in 1 ml of solution is 100 mg.
Properties of calcium chloride
Physical properties
Calcium chloride is a white solid. Cubic crystals are highly hygroscopic and quickly absorb moisture from the surrounding air. It is highly soluble in lower alcohols and organic solvents (acetone). Other physical properties:
| density | 2.15 g/cm³ |
| molar mass | 111.08 g/mol |
| boiling temperature | 1935 °C |
| melting temperature | 772 °C |
| solubility in water | 74.5 g/100 ml |
Chemical properties
Calcium chloride exhibits all the typical properties of salts.
| Property | The equation |
| Subject to reversible hydrolysis. Dissociates into calcium cations and chloride anions. | CaCl2↔Ca2+ + 2Cl- |
| Interacts with aqueous solutions of alkalis. | CaCl2 + 2NaOH(conc) →Ca(OH)2↓ + 2NaCl |
| They react chemically with strong acids. | CaCl2 + H2SO4(conc) → CaSO4↓ + 2HCl↑ |
| It enters into chemical reactions with other salts provided that one of the products precipitates or evaporates. Otherwise, the reaction will be reversible. | CaCl2+ Na2CO3 → CaCO3↓ + 2NaCl |
| It interacts with hydrogen when heated to 600-700°C and in the presence of catalysts - Pt, Ni, Fe. | CaCl2 + H2↔ CaH2 + 2HCl |
How does calcium chloride work?
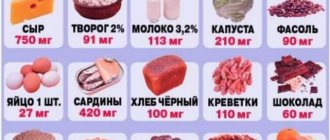
The product exhibits anti-inflammatory, anti-allergic, detoxifying properties, stabilizes the blood formula, helps strengthen vascular walls, and reduces their permeability. Calcium in the drug ensures normal functioning of the muscles and heart, regulates the conduction of nerve impulses, bone tissue synthesis, increases resistance to infections, participates in the absorption of vitamins and minerals, and maintains the necessary balance of electrolytes. Calcium chloride also normalizes the condition of the kidneys, somewhat increases diuresis, relieves and prevents swelling, helps restore the functioning of the adrenal glands, and promotes the release of adrenaline.
After use, about half the dose of the drug binds to blood proteins. Part of it is absorbed by the body. The transformation of calcium chloride occurs in the liver, the excess is excreted in the intestinal contents and urine. The intensity of excretion and absorption of the drug depends on age-related changes, dietary habits, the presence of calcium-containing foods in the diet, and hormonal status.
Preparation of a chemical compound
Calcium chloride does not occur in free form in nature. It is synthesized chemically.
Natural Sources
In nature, calcium chloride occurs exclusively in the form of crystalline hydrate with the chemical formula CaCl2 6H2O. Under the influence of high temperatures when heated above 29.8°C, crystalline hydrate loses water molecules.
Artificial sources of obtaining
In laboratory conditions, calcium chloride is synthesized by reacting calcium hydroxide with ammonium chloride.
2NH4Cl + Ca(OH)2 → 2NH3↑ + CaCl2 + 2H2O
The chemical reaction discussed underlies the industrial production of soda. The second reaction product is ammonia, and water is released as a by-product.
An alternative method is to treat calcium-containing raw materials (crystalline calcium carbonate) with hydrochloric acid.
CaCO3 + 2HCl → CaCl2 + CO2↑ + H2O
Indications for use
It is recommended to use a 10% solution of the drug:
- with calcium deficiency caused by malnutrition, systemic diseases, endocrine disorders, intestinal disorders;
- when there is a need for increased doses of calcium: age-related changes, periods of active growth, postpartum complications, general physical exhaustion;
- for bleeding of various origins;
- with nutritional edema;
- for hepatitis;
- with rickets, osteomalacia;
- for tuberculosis;
- for kidney inflammation;
- for allergic reactions;
- in case of intoxication with fluorine, magnesium, oxalic acid preparations.
During childbirth, calcium chloride is necessary to prevent eclampsia and to stimulate contractile activity of the uterus.
Application of calcium chloride
Calcium chloride is actively used in medicine, industry, and food production. The substance is used: • as a raw material for the production of calcium; • as a filler for drying structures in the form of tubes (for isolating water vapor and drying gaseous substances); • at gas distribution stations; • for quick setting of cement; • as a remedy for ice; • for regulating water hardness in the production of beverages; • as a preservative; • as a hardener in food products (health-safe additive E509); • to soften meat; • as a medicine to replenish calcium deficiency in the body; • in medicine for the treatment of allergies, toxic poisoning, hepatitis, to strengthen the walls of blood vessels, improve heart function.
When is calcium chloride contraindicated?
The injection drug should not be used in cases of increased blood clotting, thrombosis, vascular atherosclerosis, or in conditions of hypercalcemia. Pregnancy and breastfeeding are also contraindications. An exception may be an individual indication when the benefit to the mother outweighs the risks to the child.
It is necessary to refuse therapy if you have an allergic reaction. Its possible signs: increased swelling, severe skin itching following administration, bronchospasm, suffocation.
Side effects
When an injection is administered, the following body reactions are likely:
- burning pain along the veins;
- redness of the skin of the face, a feeling of heat in the mouth, face, neck and throughout the body;
- decreased heart rate, arrhythmia;
- pain in the stomach, abdominal cavity;
- nausea, vomiting.
Within 20–30 minutes after the medicine enters the bloodstream, the discomfort weakens and passes. If the injection is administered too quickly, cardiac problems may occur.
How to use calcium chloride according to instructions
In most cases, calcium chloride injections are prescribed by infusion: through droppers, at a rate of no more than 6 drops per minute. This way the drug causes fewer painful side effects. Jet injection is practiced less frequently. Intravenous injections are given slowly: administering the dose over 3–5 minutes. Subcutaneous and intramuscular injections of the drug are prohibited. The product does not dissolve in soft tissues, forming compactions and foci of necrosis.
The daily dose depends on the age and physical condition of the patients:
- For adults, the drug is administered in a volume of 5–10 ml per day;
- Children are dosed from 0.5 to 4–5 ml.
It is allowed to divide the daily dose into several parts and administer them at equal time intervals. In emergency cases, it is allowed to take calcium solution orally: consume the contents of the ampoules orally:
- for children and adolescents, the maximum dose is about 5 ml of the drug at a concentration of 10%;
- Adults can drink about 10–15 ml of the product per day.
The exact scheme, as well as the duration of the course of therapy, is determined individually.
Intravenous infusion of calcium chloride
Calcium chloride injections are used in cardiopulmonary resuscitation where hyperkalemia or hypocalcemia or calcium channel block toxicity occurs. These injections are also used to treat hypocalcemia and calcium deficiency conditions (a decrease in plasma calcium concentration below the normal range of 2.15-2.60 mmol/L) resulting from impaired or decreased absorption from the gastrointestinal tract, increased bone deposition, or when its excessive losses, for example, during breastfeeding. In addition, hypocalcemia may develop during transfusions using citrated blood or during long-term parenteral nutrition if prophylactic calcium supplements are not used. Other causes of hypocalcemia include decreased parathyroid hormone activity, vitamin D deficiency, and hypomagnesemia.
During cardiopulmonary resuscitation, a single dose of 10 ml (10%) should be administered in accordance with the algorithm recommended by the European Resuscitation Council and the Resuscitation Council (UK). For adults with acute hypocalcemia, the typical dose is 2.25 to 4.5 mmol (approximately 3 to 7 mL of 10% solution) calcium given by slow intravenous infusion and repeated as needed. This medicine is not recommended for children.
Contraindications
Hypersensitivity to the active substance or any of the excipients. During cardiac resuscitation, the use of calcium is contraindicated in the presence of ventricular fibrillation. Calcium chloride is also contraindicated in patients with conditions associated with hypercalcemia and hypercalcuria (eg, some forms of malignancy), or in patients with conditions associated with elevated vitamin D levels (eg, sarcoidosis), or in patients with kidney stones. Parenteral calcium therapy is contraindicated in patients receiving cardiac glycosides because calcium potentiates the effects of digitalis glycosides on the heart and may precipitate digitalis toxicity. Calcium chloride, due to its acidifying nature, is not suitable for the treatment of hypocalcemia caused by renal failure or in patients with respiratory acidosis or failure.
Introduction Features
Too rapid intravenous administration may result in symptoms of hypercalcemia. The use of calcium chloride is not advisable in patients with respiratory acidosis or respiratory failure due to the acidifying nature of the salt. A mild drop in blood pressure due to vasodilation may accompany the injection. Calcium chloride injection irritates the veins and should not be injected into the tissue as severe necrosis and swelling may occur. Great care should be taken to avoid extravasation or accidental injection into perivascular tissues. If perivascular infiltration occurs, intravenous infusion in this area should be stopped immediately. Local infiltration of the affected area with 1% procaine hydrochloride, to which hyaluronidase may be added, often reduces venospasm and locally dilutes calcium remaining in the tissue. Topical application of heat may also be helpful. It is especially important to prevent high concentrations of calcium from entering the heart due to the risk of fainting. When injecting into the ventricular cavity during cardiac resuscitation, care must be taken to avoid injection into myocardial tissue. Solutions must be warmed to body temperature. Injections should be given slowly through a small needle into a large vein to minimize venous irritation and avoid unwanted reactions. Rapid intravenous injections may cause the patient to complain of tingling, calcium taste, and a feeling of oppression or “hotness.”
Pharmacodynamic properties
Calcium ions increase the force of myocardial contraction. In response to electrical stimulation of muscles, calcium ions enter the sarcoplasm from the extracellular space. Calcium ions contained in the sarcoplasmic reticulum are rapidly transported to the interaction sites between the actin and myosin filaments of the sarcomere to initiate myofibril shortening. Thus, calcium increases myocardial function. The positive inotropic effects of calcium are modulated by its effect on systemic vascular resistance. Calcium can increase or decrease systemic vascular resistance. In the normal heart, the positive inotropic and vasoconstrictive effects of calcium cause a predictable increase in systemic blood pressure.
Calcium chloride in cosmetics
The drug solution is popular as part of masks for deep cleansing of facial skin. You can use them at home:
- Apply the contents of the ampoule with a cotton swab to clean skin, repeat the procedure several times after each layer has dried;
- wash your hands with solid, fragrance-free toilet soap and apply foam to your face;
- rub the soap over the skin until characteristic pellets appear under the palms;
- Continue massaging until the entire mass is peeled off the skin.
This “rolling” removes dead particles of the epidermis, dissolves remaining sebum, softens and eliminates various imperfections, and narrows pores. Regular use normalizes sebum secretion and eliminates inflammatory processes. The mask is especially useful for dull, oily and problematic skin.