General information
Homocysteine is a product of the metabolism of cysteine and methionine. Its metabolism requires folic acid, B6 and B12. If they are deficient, the level of Homocysteine may increase. An excess of this substance leads to the development of serious health problems. Blood clots and damage to vessel walls may occur. Blood test for homocysteine
is often used to determine the likelihood of cardiovascular pathologies, but its value in this regard is questionable. Studies have found that replenishing folic acid and vitamin deficiencies does not reduce the risk of disease.
A sharp increase in indicators is often observed with homocysteinuria. This is a genetic disease in which there is a defect in the enzyme that prevents the breakdown of methionine. Amino acids accumulate in the body. A newborn with this disease is visually no different from healthy babies. Violations appear only after a few years. Children with this disease are thin and tall, and have thin fingers. They develop skeletal abnormalities, develop osteoporosis, and may develop early cardiovascular diseases.
Introduction
Homocysteine is a product of the conversion of methionine, one of the eight essential amino acids. From homocysteine, another amino acid, cysteine, which is not one of the essential amino acids, can subsequently be formed.
Excess homocysteine that accumulates in the body can be converted back into methionine. Cofactors for enzymes in the methionine metabolic pathways in the body are vitamins, the most important of which are folic acid, pyridoxine (vitamin B6), cyanocobalamin (vitamin B12) and riboflavin (vitamin B1).
Homocysteine is not a structural element of proteins, and therefore does not enter the body with food. Under physiological conditions, the only source of homocysteine in the body is the conversion of methionine.
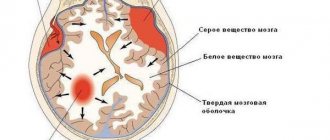
Homocysteine has a pronounced toxic effect on the cell. To protect the cell from the damaging effects of homocysteine, there are special mechanisms for removing it from the cell into the blood. Therefore, if an excess of homocysteine appears in the body, it begins to accumulate in the blood, and the main place of the damaging effect of this substance becomes the inner surface of the blood vessels.
To convert excess homocysteine to methionine, high concentrations of the active form of folic acid (5-methyltetrahydrofolate) are required. The main enzyme that converts folic acid into its active form is 5,10 methylenetetrahydrofolate reductase (MTHFR). A decrease in the activity of this enzyme is one of the important reasons for the accumulation of homocysteine in the body. (Fig. 1)
Rice. 1
Please note that homocysteine can only be formed from methionine. Homocysteine can be converted either to cystathionine, which is subsequently used for the synthesis of cysteine, or to methionine. Vitamins play an important role in all key stages of methionine and homocysteine metabolism. Red indicates the methionine conversion cycle, green indicates the folic acid conversion cycle. MTHFR - 5,10 methylenetetrahydrofolate reductase. Methionine, homocysteine and folic acid are amino acids. Amino acids are the most important substrates for nitrogen metabolism in the body. Proteins, enzymes, purine and pyrimidine bases (and nucleic acids), pyrrole derivatives (porphyrins), biologically active compounds of peptide nature (hormones), as well as a number of other compounds originate from amino acids. When needed, amino acids can serve as a source of energy, mainly through the oxidation of their carbon skeleton. In living organisms, amino acids form a pool, the value of which in adulthood remains constant under physiological conditions. It corresponds to the difference between the supply of amino acids from the outside or sometimes from endogenous sources, and the consumption of amino acids that serve as substrates in anabolic and catabolic processes. Living organisms do not store amino acids and proteins for future use, so the required amount of nitrogen (preferably in the form of amino acids) must come from food. In an adult body under physiological conditions, the amount of nitrogen entering and exiting is the same (nitrogen balance). Amino acids from exogenous sources (from food) are absorbed in the digestive tract and transported by the blood to the liver and other tissues and organs, where they are further used. In addition, the source of amino acids (endogenous source) can be tissue proteins of the body, which are constantly subject to metabolism to release the amino acids they contain. These amino acids are used to a small extent for the synthesis of new proteins, but endogenous sources are very important because they provide about two-thirds of the total amino acid pool, and only one-third of the amino acids come from food. Essential amino acids are those amino acids that cannot be synthesized by a given body. For humans, these are valine, leucine, isoleucine, lysine, methionine, threonine, phenylalanine, tryptophan and, under certain conditions, also arginine and histidine.
Interpretation of results
Reference values vary depending on age. To correctly interpret the results, you must consult a doctor; self-diagnosis is unacceptable. Elevated levels of homocysteine may indicate a deficiency of B vitamins, kidney failure, diabetes, psoriasis, senile dementia, and pregnancy complications. They can also be elevated due to malnutrition. Significantly elevated levels in a newborn indicate homocysteinuria. To confirm this diagnosis, additional examination is required. Amino acid deficiency is observed in Down syndrome and hyperthyroidism.
The test results may be affected by long-term use of a number of medications, so you should inform your doctor if you are taking any medications.
References
1. Clinical aspects of hyperhomocysteinemia: monograph / V.A. Snezhitsky [and others]; under general editorship V.A. Snezhitsky, V.M. Pyrochkina. – Grodno: GrSMU, 2011. – 292 p. 2. Kostyuchenko G.I. Hyperhomocysteinemia: clinical significance, age-related characteristics, diagnosis and correction // Clinical gerontology. 2007. No. 4. URL: https://cyberleninka.ru/article/n/gipergomotsisteinemiya-klinicheskoe-znachenie-vozrastnye-osobennosti-diagnostika-i-korrektsiya (date of access: 08/30/2020). 3. EC/EOA GUIDELINES FOR THE DIAGNOSIS AND TREATMENT OF DYSLIPIDEMIAS 2021 Original publication European Heart Journal (2016), 37 (39): 2999-3058, doi:10.1093/eurheartj/ehw272
Hyperhomocysteinemia and pregnancy pathology
Hyperhomocysteinemia leads to damage and activation of endothelial cells (the cells lining blood vessels), which significantly increases the risk of developing thrombosis. Not all details of the mechanism of the pathological action of hyperhomocysteinemia have been fully studied, but much is already known.
The thrombogenic effect of homocysteine may be associated with damage to endothelial cells, nonspecific inhibition of prostacyclin synthesis, activation of factor V, inhibition of protein C activation, down-regulation of thrombomodulin expression, blockade of tissue plasminogen activator binding by endothelial cells. In addition, high homocysteine levels increase platelet aggregation due to decreased endothelial synthesis of relaxing factor and NO, induction of tissue factor, and stimulation of smooth muscle cell proliferation.
Microthrombi formation and microcirculation disorders lead to a number of obstetric complications. Disorders of placentation and fetoplacental circulation can cause reproductive failure: miscarriage and infertility as a result of defects in embryo implantation. In later stages of pregnancy, hyperhomocysteinemia causes the development of chronic fetoplacental insufficiency and chronic intrauterine fetal hypoxia. This leads to the birth of children with low body weight and a decrease in the functional reserves of all life-supporting systems of the newborn and the development of a number of complications during the newborn period.
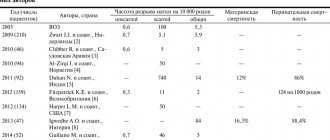
Hyperhomocysteinemia may be one of the reasons for the development of generalized microangiopathy in the second half of pregnancy, manifested in the form of late toxicosis (preeclampsia): nephropathy, preeclampsia and eclampsia. Hyperhomocysteinemia is characterized by the development of severe, often uncontrollable conditions that can lead to early termination of pregnancy for medical reasons. The birth of an immature premature baby in such cases is accompanied by high infant mortality and a high percentage of neonatal complications.
Homocysteine freely crosses the placenta and can have teratogenic and fetotoxic effects. Hyperhomocysteinemia has been shown to be one of the causes of anencephaly and spina bifida.
).
Anencephaly has a 100% mortality rate, and spina bifida
causes serious neurological problems in the child, including motor paralysis, lifelong disability and premature death. A direct toxic effect of excess homocysteine levels on the fetal nervous system cannot be excluded.
Hyperhomocysteinemia can be not only the cause, but also a companion to obstetric complications. It is assumed that in some cases problems may be associated not only with high homocysteine levels, but also with those conditions that cause the development of hyperhomocysteinemia (vitamin deficiency conditions, concomitant diseases, etc.)
It should be remembered that hyperhomocysteinemia can be accompanied by the development of secondary autoimmune reactions and is currently considered as one of the causes of antiphospholipid syndrome. Autoimmune factors can interfere with the normal development of pregnancy even after high homocysteine levels are eliminated.
What else should you know?
If the results of laboratory tests confirm the diagnosis of homocystinuria, then in some cases genetic testing is performed. Genetic testing is ordered to identify one or more of the most common genetic mutations. If the patient has an unfavorable family history of early atherosclerosis or one of the family members has been diagnosed with homocystinuria, then the patient should be tested for the genetic mutation that was found in the family member.
Homocysteine levels can increase with age, as well as with smoking, drinking alcohol, drinking large amounts of coffee, or using drugs such as carbamazepine, methotrexate, or phenytoin. Its concentration in women is lower than in men, but increases after menopause, possibly due to a decrease in estrogen production.
Homocysteine levels may increase in some diseases, for example, hypothyroidism, renal failure, proliferative diseases.
Diagnosis of hyperhomocysteinemia
To diagnose hyperhomocysteinemia, the level of homocysteine in the blood is determined. For the differential diagnosis of various forms of homocysteinemia, stress tests with methionine are sometimes used (determining the level of homocysteine on an empty stomach and after a methionine load).
To find out the causes of hyperhomocysteinemia, DNA diagnostics of hereditary defects in enzymes involved in the metabolism of methionine and folic acid, in particular MTHFR, and determination of the level of vitamins B6, B12, B1 and folic acid in the blood are carried out.
If high levels of homocysteine in the blood are detected, tests are recommended to exclude additional risk factors for the development of vascular and obstetric complications. We recommend a hemostasiogram, a blood test for lupus anticoagulant, a test for antiphospholipid and anti-DNA antibodies, anti-thyroid antibodies, anti-nerve growth factor antibodies, and a blood test for hereditary hemostatic defects (Leiden mutation and hereditary prothrombin defects). Other tests may be prescribed according to indications.