Home | About us | Delivery | Advertisers | Login | Registration
Delivery on Sundays and holidays does not work!
- Medicines
- dietary supplementsVitamins
- Categories from A to Z
- Brands from A to Z
- Products from A to Z
- Medical equipment
- beauty
- Child
- Care
- Honey products appointments
- Herbs and herbal teas
- Medical nutrition
- Journey
- Making medicinesStock
Pharmacy online is the best pharmacy in Almaty, delivering medicines to Almaty. An online pharmacy or online pharmacy provides the following types of services: delivery of medicines, medicines to your home. Online pharmacy Almaty or online pharmacy Almaty delivers medicines to your home, as well as home delivery of medicines in Almaty.
my basket
Apteka84.kz is an online pharmacy that offers its customers medicines, medicinal and decorative cosmetics, dietary supplements, vitamins, baby food, intimate products for adults, medical equipment and thousands of other medical and cosmetic products at low prices. All data presented on the Apteka84.kz website is for informational purposes only and is not a substitute for professional medical care. Apteka84.kz strongly recommends that you carefully read the instructions for use contained in each package of medicines and other products. If you currently have any symptoms of the disease, you should seek help from a doctor. You should always tell your doctor or pharmacist about all the medicines you take. If you feel you need further help, please consult your local pharmacist or contact our GP online or by telephone.
© 2021 Pharmacy 84.
Chorionic gonadotropin (vial 500 units No. 5 + solution)
A country
The country of production may vary depending on the batch of goods. Please check with the operator for detailed information when confirming your order.
Active substance
Chorionic gonadotropin
Compound
The active substance is human chorionic gonadotropin.
pharmachologic effect
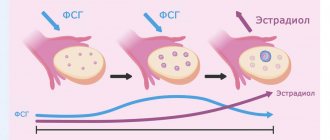
Human chorionic gonadotropin (hCG) is a gonadotropic hormone that is produced by the placenta during pregnancy and then excreted unchanged by the kidneys. To obtain the drug, it is extracted from urine and purified. Necessary for normal growth and maturation of gametes in women and men, as well as for the production of sex hormones. Pharmacodynamics. It has a gonadotropic, follicle-stimulating and luteinizing effect. Luteinizing activity prevails over follicle-stimulating activity. Stimulates the development of genital organs and secondary sexual characteristics. In women, the drug causes ovulation and stimulates the synthesis of estrogens (estradiol) and progesterone. In men, it stimulates spermatogenesis, the production of testosterone and dihydrotestosterone. Pharmacokinetics: After intramuscular administration, it is well absorbed. The half-life is 8 hours. The maximum concentration of hCG in the blood plasma is achieved after 4-12 hours. The half-life of human chorionic gonadotropin is 29-30 hours; in the case of daily intramuscular injections, accumulation of the drug may be observed. Chorionic gonadotropin is excreted by the kidneys. About 10-20% of the administered dose is found unchanged in the urine, the main part is excreted in the form of beta chain fragments.
Indications for use
In women: ovarian dysfunction (anovulatory), amenorrhea; maintenance of the corpus luteum phase. In men and boys: hypogonadotropic hypogonadism; delayed puberty due to insufficiency of the gonadotropic function of the pituitary gland; cryptorchidism not caused by anatomical obstruction; insufficiency of spermatogenesis, oligo-asthenospermia, azoospermia; when conducting a differential diagnostic test for anorchidism and cryptorchidism in boys; when conducting a functional Leydig test to assess testicular function in hypogonadotropic hypogonadism before starting long-term stimulating treatment.
Mode of application
After adding the solvent to the lyophilisate, the reconstituted solution of human chorionic gonadotropin is administered intramuscularly, slowly. The prepared solution cannot be stored, since further preservation of the sterility of the solution is not guaranteed. The indicated dosages are approximate, treatment should be adjusted by the doctor individually depending on the required response to the administration of the drug. In women: for anovulatory cycles, human chorionic gonadotropin is prescribed starting from the 10-12th day of the menstrual cycle, 3000 IU 2-3 times with an interval of 2-3 days or 1500 IU 6-7 times every other day; to maintain the corpus luteum phase, two to three repeated injections of the drug can be given at a dose of 1500 IU to 5000 IU each within 9 days after ovulation or embryo transfer (for example, on 3, 6 and 9th day after ovulation induction). In men and boys: with hypogonadotropic hypogonadism - 1000-2000 IU of the drug 2-3 times a week. In case of infertility, it is possible to combine human chorionic gonadotropin with an additional drug containing follitropin (follicle-stimulating hormone) 2-3 times a week. The course of treatment should continue for at least 3 months before any improvement in spermatogenesis can be expected. Testosterone replacement therapy must be suspended during this treatment. When improvement in spermatogenesis is achieved, to maintain it, in some cases, isolated use of human chorionic gonadotropin is sufficient; for delayed puberty due to insufficiency of the gonadotropic function of the pituitary gland - 1500 IU 2-3 times a week. The course of treatment is at least 6 months; for cryptorchidism not caused by anatomical obstruction: at the age of 3 to 6 years - 500-1000 IU twice a week for 6 weeks; at the age of over 6 years - 1500 IU twice a week for 6 weeks. The course of treatment, if necessary, can be repeated; if spermatogenesis is insufficient, oligoasthenospermia, azoospermia, 500 IU in combination with menotropin (75 IU follicle-stimulating hormone + 75 IU luteinizing hormone) is prescribed daily, or 2000 IU every 5 days in combination with menotropin (150 IU follicle-stimulating hormone + 150 IU luteinizing hormone) 3 times a week for 3 months. If there is no response to treatment, 2000 IU is prescribed 2-3 times a week with menotropin (150 IU follicle-stimulating hormone + 150 IU luteinizing hormone) 3 times a week for 3-12 months. When an improvement in spermatogenesis is achieved, subsequent therapy in some cases can be carried out only with maintenance doses of human chorionic gonadotropin; for the purpose of differential diagnosis of cryptorchidism and anorchism in boys, human chorionic gonadotropin is administered intramuscularly in a single dose of 100 IU/kg, the concentration of testosterone in the blood serum is determined before the test and after 72 -96 hours after injection of the drug. In the case of anorchidism, the test will be negative, indicating the absence of testicular tissue; in the case of cryptorchidism, even if only one testicle is present, the test will be positive (5-10-fold increase in testosterone concentration). If the test is weakly positive, a search for the gonad (abdominal ultrasound or laparoscopy) is necessary, as there is a high risk of malignancy.
Interaction
It is necessary to avoid combined use of the drug with high doses of glucocorticosteroids. No other interactions with drugs have been noted.
Side effect
Immune system disorders: In rare cases, a generalized rash or fever may occur. General disorders and injection site conditions: When using human chorionic gonadotropin, reactions at the injection site, such as bruising, pain, redness, swelling and itching, may occur. Allergic reactions have been reported in some cases, most of which include pain and/or rash at the injection site; increased fatigue. In women. Metabolic and nutritional disorders: edema. Mental disorders: irritability, anxiety, depression. Nervous system disorders: headache, dizziness. In men and boys. Endocrine system disorders: premature puberty Disorders of the skin and subcutaneous tissues: acne. Disorders of the genital organs and breast: treatment with human chorionic gonadotropin can sporadically cause gynecomastia; prostatic hyperplasia, enlarged penis, increased sensitivity of the nipples of the mammary glands in men, enlarged testicles in the inguinal canal with cryptorchidism.
Contraindications
Hypersensitivity to hCG or to any component of the drug; hormone-dependent malignant tumors of the genital organs and breast currently or suspected of them (ovarian cancer, breast cancer, uterine cancer in women and prostate cancer, breast carcinoma in men); organic lesions of the central nervous system (CNS) (tumors of the pituitary gland, hypothalamus); deep vein thrombophlebitis; hypothyroidism; adrenal insufficiency; hyperprolactinemia; children under 3 years of age. In boys: precocious puberty. In men: infertility not associated with hypogonadotropic hypogonadism. In women: abnormal formation of the genital organs, incompatible with pregnancy; fibrous tumor of the uterus, incompatible with pregnancy; primary ovarian failure; infertility not associated with anovulation (for example, tubal or cervical origin); bleeding or spotting from the vagina of unknown etiology; pregnancy and period breastfeeding.
Overdose
The drug is characterized by extremely low toxicity. In women with an overdose, ovarian hyperstimulation syndrome (OHSS) may occur. Depending on the severity (based on clinical and laboratory symptoms), several types of OHSS are distinguished. Mild OHSS: abdominal discomfort, mild abdominal pain, ovarian size is usually less than 8 cm; moderate OHSS: breast tenderness, moderate abdominal pain, nausea and/or vomiting, diarrhea, ultrasound signs of ascites, slight or moderate enlargement of ovarian cysts, ovarian size is usually 8 -12 cm; Severe OHSS: weight gain, in rare cases thromboembolism, clinical signs of ascites (sometimes hydrothorax), oliguria, hemoconcentration, hematocrit greater than 45%, hypoproteinemia, large ovarian cysts (prone to rupture), ovarian size, usually , more than 12 cm. Principles of treatment of OHSS. Mild degree: bed rest; drinking plenty of mineral water; monitoring the patient’s condition. Moderate and severe (only in a hospital setting): monitoring the function of the cardiovascular system (CVS), respiratory system, liver, kidneys, electrolyte and water balance (diuresis, weight dynamics, changes in abdominal circumference) ; control of hematocrit level; intravenous crystalloid solutions (to restore and maintain circulating blood volume (BCV)); intravenous colloid solutions - 1.5-3 l/day (while maintaining hemoconcentration) and persistent oliguria; hemodialysis (with the development of renal failure ); corticosteroids, antiprostaglandins, antihistamines (to reduce capillary permeability); for thromboembolism - low molecular weight heparins (fraxiparine, clexane); plasmapheresis - 1-4 sessions with an interval of 1-2 days (improving the rheological properties of blood, normalizing the acid-base state ( CBS) and blood gas composition, reduction in ovarian size); paracentesis and transvaginal puncture of the abdominal cavity for ascites. Hospitalization is necessary for the development of moderate and severe OHSS. Men and boys may develop gynecomastia; in boys, behavioral changes similar to those observed during the first phase of puberty are possible; degeneration of the gonads (with unreasonably long-term use for cryptorchidism), atrophy of the seminiferous tubules (due to inhibition of the production of follicle-stimulating hormone (FSH) as a result of stimulation of the production of androgens and estrogens); decrease in the number of sperm in the ejaculate (if the drug is abused in men). Long-term use of the drug may lead to increased side effects.
special instructions
With caution. Persons with risk factors for thrombosis (personal or family history, severe obesity (body mass index >30 kg/m2) or thrombophilia). In men and boys with latent or overt heart failure, renal dysfunction, arterial hypertension, epilepsy or migraine (or a history of these conditions); in prepubertal boys; in patients with bronchial asthma. If you have one of the listed diseases, be sure to consult your doctor before taking the drug. Use during pregnancy and breastfeeding. Use of the drug during pregnancy and breastfeeding is contraindicated. The use of gonadotropin increases the risk of developing venous or arterial thromboembolism Therefore, it is necessary to evaluate the benefits of in vitro fertilization therapy for patients at risk. It should also be noted that pregnancy itself is also accompanied by an increased risk of thrombosis. The likelihood of multiple pregnancies increases. During treatment with the drug and for 10 days after stopping treatment, human chorionic gonadotropin may affect the values of immunological tests for the concentration of hCG in the blood plasma and urine, which can lead to a false-positive pregnancy test result. Treatment of male patients with human chorionic gonadotropin leads to increased production of androgens, therefore, patients at risk should be under strict medical supervision, since exacerbation of the disease or relapse can sometimes be the result of increased production of androgens. HCG contributes to premature closure of the epiphyses or premature puberty. It is necessary to regularly monitor skeletal development. In men, the drug is ineffective with high levels of follicle-stimulating hormone. Unreasonably long-term use of the drug for cryptorchidism, especially if surgical intervention is indicated, can lead to degeneration of the gonads. Long-term administration can lead to the formation of antibodies to the drug. Impact on the ability to drive vehicles and machines. During the treatment period, you must refrain from driving vehicles and engaging in potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Dispensing conditions in pharmacies
On prescription