YOU MAY ALSO LIKE
Newborn care
Fever in babies and toddlers: recommendations and guidelines for action
The rosy cheeks of a baby usually only cause tenderness. True, sometimes pink cheeks are one of the symptoms of the disease. Fifth disease, or erythema infectiosum, is a viral disease, one of the manifestations of which is a rash on the face of a child. It can be mistaken for blush on the cheeks. As a rule, this disease is not dangerous, but it is worth learning more about it in case your baby becomes infected with it. In this article we will tell you about the fifth disease, its manifestations and symptoms, methods of prevention, and when you need to see a doctor.
What is fifth disease?
Fifth disease is an infectious disease caused by parvovirus B19. Most often it affects children aged 5 to 15 years. This disease is considered mild because children mostly feel well, even if they develop a rash. The medical name is erythema infectiosum, and the phrase “fifth disease” arose because erythema was fifth on the list of six common childhood diseases accompanied by a rash. Here is a list of these diseases:
- measles
- scarlet fever
- rubella
- Infectious mononucleosis
- erythema infectiosum
- roseola infantile (sudden exanthema)
Fortunately, having recovered from the fifth disease, your child will forever acquire immunity to it.
What are the symptoms and manifestations of fifth disease?
The main symptom of fifth disease is a rash. As a rule, it does not appear at the very beginning of the disease, but after a couple of days. First it appears on the face. After a few days, the rash spreads to the child's arms, torso, thighs, buttocks, and feet. In adults, the rash usually does not appear. The rash looks like pink spots that can merge with each other in the form of a kind of “lace”. Elements of the rash can be either true spots (that is, not felt by the fingers), or have edges slightly raised above the skin level. Often such satiety is accompanied by itching, especially severe in the area of the feet. The rash usually goes away within seven to ten days. It sometimes occurs again weeks or months later, often after exposure to sunlight, extreme heat, or extreme cold. At the very beginning of the disease, even before the rash appears, fifth disease can manifest itself with symptoms similar to those of the common cold:
- a sore throat
- headache
- stuffy or runny nose
- redness of the eyes
- weakness
- elevated temperature
- diarrhea
- indigestion
- itching
- enlarged lymph nodes
- joint pain or swelling (hands, wrists, knees, ankles), although this is more common in older children and adults
How to make an appointment with an allergist
You can make an appointment with a specialist from JSC “Medicine” (clinic of Academician Roitberg) on the website through a special feedback form. You can also call +7. We are located in the Central Administrative District, at the address: 2nd Tverskoy-Yamskoy lane 10. The metro station is located nearby. Mayakovskaya, metro station Belorusskaya, metro station Novoslobodskaya, Tverskaya metro station, Chekhovskaya metro station.
An allergist will perform a visual examination, take into account the severe symptoms and conduct a number of research activities. Based on the data obtained, a diagnosis will be made and treatment will be prescribed. After completion of therapy, a repeat study will be performed.
How long can fifth disease be contagious?
The fifth disease is transmitted to other people within a week from the onset of the disease (that is, the appearance of the first symptoms similar to the manifestations of ARVI) until the appearance of a rash in the child. Once the rash appears, the child is no longer contagious. Just in case, do not allow your baby to come into contact with other people, even when he has a rash, especially if he has a fever. This way the virus will not spread further. If your baby has erythema infectiosum, try to keep him away from pregnant women. If an expectant mother becomes infected with this virus during pregnancy, it can lead to serious problems in the development of the fetus.
Parvovirus infection B19
In 1975, Yvonne Cossart, while studying donor serum in sample No. 19 of line B, identified parvovirus-like particles, called “Parvovirus B19” [1]. Parvovirus B19 (erythrovirus B19) - contains single-stranded DNA, a non-enveloped, thermostable virus, 20-hedron shaped, 20–25 nm in diameter, belongs to the genus Erythrovirus, family Parvoviridae (Fig. 1). There are three genotypes: genotype 1 is ubiquitous; genotype 2 is quite rare in Europe, Brazil, Vietnam, North America; genotype 3 is predominantly reported in western Africa, with sporadic cases reported in France, Great Britain, Brazil and Asia; in South Africa, all three genotypes are recorded [2–4].
The parvovirus B19 genome encodes: two capsid proteins VP1 and VP2; non-structural protein NS-1; small non-structural proteins 11-Da, 9-Da and 7.5-kDa. Protein NS-1 is a transactivator of the viral P6 promoter of WAF1/CIP1 gene expression, the product of which p21WAF inhibits cyclin-Cdk complexes, blocking the cell cycle in the G1 phase; NS-1 also disrupts the function of the E2 F-family of transcription factors, blocking the cell cycle in G2 /M phase, which ensures viral DNA replication [5–7]. The VP2 protein has the ability to self-assemble the viral capsid in the absence of viral DNA, forming particles similar in antigenic and immunogenic properties to the virion [8]. The VP1 protein contains a phospholipase A2-like region (VP1 u), which is responsible for virus invasion into the nucleus of an infected cell [9]. 11-Da protein plays an important role in viral DNA replication and apoptosis of infected cells [10].
The cellular receptor for parvovirus B19 is the P-antigen, located on the cells of trophoblast, bone marrow, liver, kidney, lung, synovium, epithelium, endothelium, myocytes, and lymphoid tissue [11, 12]. People who lack P antigen (1 in 200,000 people) are not susceptible to parvovirus infection [13]. The life cycle of parvovirus B19 consists of the following stages: binding to P-antigen; internalization - penetration into the cell using a5b1-integrins; transition to the cell nucleus, DNA replication; transcription, capsid assembly; packaging of the genome into the capsid, lysis of the host cell and release of virions [14]. The main components of the cellular and humoral immune response to parvovirus B19 are presented in Fig. 2 [15].
Parvovirus B19 is widespread in the human population, more than 80% of the adult population are seropositive, the frequency of detection of IgG to parvovirus in children in the first ten years of life ranges from 2% to 21% (Fig. 3). In 25–68% of cases, parvovirus B19 infection is asymptomatic [16]. During pregnancy, 1–5% of women are susceptible to parvovirus infection; during the period when the incidence rises to 3–34%, in 50% of women parvovirus infection is asymptomatic [17, 18]. The maximum level of P-antigen on trophoblast villi is recorded in the first and second trimester of pregnancy, which determines transplacental transmission of the virus with the development of spontaneous abortions, non-immune hydrops, and intrauterine fetal death [19, 20].
The main route of transmission of parvovirus B19 infection is airborne; transplacental, transfusion, and transplantation routes of infection are also possible [21–23]. Winter-spring seasonality is typical, with a maximum increase in incidence in the spring months. Epidemic increases in the incidence of parvovirus infection are observed every 3–6 years. The incubation period averages from 4 to 14 days (maximum up to 21 days), viremia develops approximately 7 days after virus inoculation and continues for 4–7 days [24]. The pathophysiological components of parvovirus B19 infection are presented in Fig. 4.
Parvovirus B19 is associated with a variety of diseases, the development of which is associated with the characteristics of a person’s immunological and hematological status (table).
Erythema infectiosum
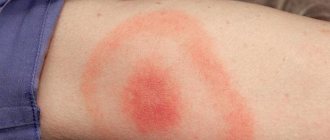
Erythema infectiosum is the most common clinical form of parvovirus B19 infection in children. It begins with nonspecific symptoms - fever, malaise, chills, myalgia, which last for 2-5 days. Then the pathognomonic symptom of “spanked” cheeks is added - bright erythema of the skin in the cheek area, as well as a maculopapular rash on the trunk and limbs, quickly transforming into a “lace” rash, itching does not bother (Fig. 5). Exanthema due to parvovirus infection can persist for up to 2–3 weeks, resolves on its own, and sometimes there is an increase in the rash after a “hot” bath or exposure to UV rays [25].
Erythema maculopapular with glove-and-sock syndrome
This form of parvovirus infection B19 is characterized by: acute onset of the disease with fever, malaise, headache, loss of appetite, arthralgia; swelling of the hands and feet with the simultaneous appearance of maculopapular erythema on the skin, in some cases with a hemorrhagic component, in the form of “gloves and socks” (Fig. 6); in the oral cavity, erosions can form on the mucous membrane of the hard and soft palate, tongue, and lips [26–28]. The duration of the disease is 1–2 weeks, symptoms resolve on their own.
Polyarthropathy syndrome
Polyarthropathy syndrome is observed in 75% of adults with erythema infectiosum, more often in middle-aged women. The process involves the metacarpophalangeal, wrist, elbow, knee, and ankle joints. Usually there is symmetrical damage to the joints. Polyarthropathy syndrome clinically resembles rheumatoid arthritis, can persist for 3 months, and recur several times during the year [29].
Transient aplastic crisis
Transient aplastic crisis develops with parvovirus B19 infection in patients with hereditary microspherocytosis, sickle cell anemia, iron deficiency anemia, and thalassemia. It is characterized not only by partial hypoplasia of the erythroid lineage, but also by the development of thrombocytopenia, neutropenia and even pancytopenia [30, 31].
CNS damage
The neurological manifestation of parvovirus B19 infection is accompanied by the development of: encephalitis, meningoencephalitis, convulsive syndrome, peripheral neuropathies, carpal tunnel syndrome [32].
Liver damage
Parvovirus B19 can cause acute hepatitis, fulminant liver failure, chronic hepatitis, hepatitis-associated aplastic anemia, hepatitis with hemophagocytic lymphohistiocytosis [33]. Liver biopsy reveals hepatocellular and canalicular cholestasis, apoptosis and necrosis of hepatocytes [34, 35].
Carditis
Until recently, enteroviruses and adenoviruses were considered the main causes of viral carditis, but with the introduction of the polymerase chain reaction method with hybridization-fluorescence detection, parvovirus B19 and herpes virus type 6 began to be increasingly recorded during endomyocardial biopsy [36]. Numerous copies of the viral genome of parvovirus B19 are determined in the endothelium of intramyocardial arterioles, capillaries and postcapillary venules, initiating the synthesis of tumor necrosis factor alpha (TNF-a), interleukins (IL): IL-6, IL-8, IL-2, interferon gamma (IFN) -g), IL-2 receptors [37]. The direct cytopathic effect of parvovirus, apoptosis, and activation of the innate and adaptive immune response lead to endothelial dysfunction with subsequent ventricular remodeling and the development of dilated cardiomyopathy [38–40].
Nonimmune hydrops fetalis
Non-immune hydrops fetalis can develop when parvovirus B19 affects a fetus with a gestational age of 13–20 weeks. Accompanied by anemia, hypoxia, hepatitis (direct damage to hepatocytes by the virus and indirect damage due to hemosiderin deposition), carditis, with the formation of liver and heart failure. An ultrasound examination of the fetus reveals: cardiomegaly, thoracic edema, ascites, fluid effusion into the pleural cavities and pericardium, placental edema [41]. With timely diagnosis and blood transfusion to the fetus in utero, a favorable outcome is possible in 83% of cases [42].
Congenital parvovirus infection B19
In the first months of a child’s life, congenital parvovirus infection manifests itself with the development of Diamond–Blackfan anemia, hydrocephalus, polymicrogyria, carditis, and hepatitis [43, 44].
Diagnosis of parvovirus infection B19
Hematological changes are often transient in the form of anemia, reticulocytopenia or complete absence of reticulocytes, neutropenia, eosinophilia, monocytosis, thrombocytopenia. There may be an increase in liver transaminases (alanine aminotransferase and aspartate aminotransferase), C-reactive protein, and erythrocyte sedimentation rate.
Methods for diagnosing parvovirus infection B19:
- Polymerase chain reaction (PCR) with hybridization-fluorescent detection "AmpliSens® Parvovirus B19-FL" (serum, cerebrospinal fluid, bone marrow punctate, skin biopsy, etc.) - determination of parvovirus DNA.
- Enzyme-linked immunosorbent assay (ELISA) “Parvovirus B19 IgM/Parvovirus B19 IgG” (blood serum) - IgM in the patient’s blood serum is detected simultaneously with the onset of symptoms of the disease (12–14 days after infection), their level reaches a maximum on the 30th day, then decreases over 2–3 months. After 5–7 days from the moment of clinical manifestations of parvovirus infection, IgG appears, which persist for several years.
- Parvovirus B19 immunoblot IgM/IgG “RIDA® Blot Parvovirus B19” (blood serum).
- Considering the ability of parvovirus B19 to isolate in tropic structures (bone marrow, trophoblasts), a negative blood PCR result for parvovirus B19 does not mean the absence of infection, but should be considered as a possible false negative. For greater diagnostic information, PCR of various materials should be performed in combination with IgM/IgG immunoblot.
If a pregnant woman exhibits symptoms of parvovirus B19 infection (erythema infectiosum, arthropathy) or has been in contact with a patient with this infection, PCR and/or serum ELISA is recommended. If IgM to parvovirus is detected or a positive PCR result is detected, fetal ultrasound is required every 2 weeks. If there are ultrasound signs of non-immune fetal hydrops, cordocentesis or amniocentesis (PCR of fetal blood or amniotic fluid for parvovirus B19) is indicated [45]. If there are positive data for parvovirus B19 and ultrasound signs of fetal damage, the question of termination of pregnancy is raised.
Therapy for parvovirus B19 infection
Currently, there is no specific etiotropic therapy for parvovirus infection. Depending on the clinical form of parvovirus B19 infection, syndromic therapy is carried out (non-steroidal anti-inflammatory drugs, glucocorticosteroid drugs, red blood cell transfusion, etc.). For damage to the nervous system, carditis, hepatitis - intravenous human immunoglobulin. The most effective immunoglobulin preparations are those obtained from a large number of donors: Octagam, Intraglobin, Pentaglobin.
Prevention of parvovirus B19 infection
There is currently no effective prevention of parvovirus infection. The development of a safe and immunogenic vaccine against parvovirus B19 infection is underway [46].
The main preventive measures are aimed at preventing intrauterine infection of the fetus: the introduction of screening before a planned pregnancy and during pregnancy for the presence of IgG to parvovirus B19; informing a seronegative pregnant woman about the existence of parvovirus B19 infection, as well as the need to limit contact with children, especially those with typical manifestations of parvovirus infection [47].
In conclusion, it should be noted that in our country there are no statistics on the incidence of parvovirus infection B19, and there is also no accurate data on cases of congenital parvovirus infection. Our experience shows that in some cases, with clinical manifestations of congenital infection, parvovirus B19 can be associated in particular with herpes viruses, which significantly complicates both the diagnosis and treatment of these patients. Taking this into account, it should be considered rational to include screening for parvovirus B19 in newborn children when a congenital infection is suspected.
Literature
- Cossart YE, Field AM, Cant B. et al. Parvovirus-like particles in human sera // Lancet. 1975; 1:72–73.
- Servant A., Laperche S., Lallemand F. et al. Genetic diversity within human erythroviruses: identification of three genotypes // Journal of Virology. 2002; 76(18):9124–9134.
- Candotti D., Etiz N., Parsyan A., Allain J.-P. Identification and characterization of persistent human erythrovirus infection in blood donor samples // J Virol. 2004; 78:12169–12178.
- Corcoran C., Hardie D., Yeats J., Smuts H. Genetic variants of human parvovirus B19 in South Africa: cocirculation of three genotypes and identification of a novel subtype of genotype 1 // J Clin Microbiol. 2010; 48: 137–142.
- Zhi N., Mills IP, Lu J. et al. Molecular and functional analyzes of a human parvovirus B19 infectious clone demonstrates essential roles for NS1, VP1, and the 11-kilodalton protein in virus replication and infectivity // J. Virol. 2006; 80:5941–5950.
- Nakashima A., Morita E., Saito S., Sugamura K. Human Parvovirus B19 nonstructural protein transactivates the p21/WAF1 through Sp1 // Virology. 2004; 329(2):493–504.
- Wan Z., Zhi N., Wong S. et al. Human parvovirus B19 causes cell cycle arrest of human erythroid progenitors via deregulation of the E2 F family of transcription factors // J. Clin. Invest. 2010; 120:3530–3544.
- Kaufmann B., Chipman PR, Kostyuchenko VA et al. Visualization of the externalized VP2 N termini of infectious human parvovirus B19 // J. Virol. 2008; 82:7306–7312.
- Zadori Z., Szelei J., Lacoste MC et al. A viral phospholipase A2 is required for parvovirus infectivity // Dev. Cell. 2001; 1: 291–302.
- Chen AY et al. The small 11 kDa non-structural protein of human parvovirus B19 plays a key role in inducing apoptosis during B19 virus infection of primary erythroid progenitor cells // Blood. 2010; 115:1070–1080.
- Brown KE, Anderson SM, Young NS Erythrocyte P antigen: cellular receptor for B19 parvovirus // Science. 1993; 262:114–117.
- Cooling LL, Koerner TA, Naides SJ Multiple glycosphingolipids determine the tissue tropism of parvovirus B19 // J Infect Dis. 1995; 172:1198–1205.
- Brown KE, Hibbs JR, Gallinella G. et al. Resistance to parvovirus B19 infection due to lack of virus receptor (erythrocyte P antigen) // NEJM. 1994; 330:1192–1196.
- Servant-Delmas A., Lefrere JJ, Morinet F. et al. Advances in human B19 erythrovirus biology // J Virol. 2010; 84:9658–9665.
- Corcoran A., Doyle S. Advances in the biology, diagnosis and host–pathogen interactions of parvovirus B19 // Journal of Medical Microbiology. 2004; 53:459–475.
- Noyola DE, Lourdes Padilla-Ruiz M., Guadalupe Obregón-Ramos M. et al. Parvovirus B19 infection in medical students during a hospital outbreak // J Med Microbiol. 2004; 53: 141–146.
- Feldman DM, Timms D., Borgida AF Toxoplasmosis, parvovirus, and cytomegalovirus in pregnancy // Clin Lab Med. 2010; 30(3):709–720.
- Tolfvenstam T., Broliden K. Parvovirus B19 infection // Semin Fetal Neonatal Med. 2009; 14 (4): 218–221.
- Jordan JA, DeLoia JA Globoside expression within the human placenta // Placenta. 1999; 20: 103–108.
- Public Health Laboratory Service Working Party on Fifth Disease. Prospective study of human parvovirus infection in pregnancy // Br Med J. 1990; 300:1166–1170.
- Jordan J., Tiangco B., Kiss J., Koch W. Human parvovirus B19: prevalence of viral DNA in volunteer blood donors and clinical outcomes of transfusion recipients // Vox Sang. 1998; 75:97–102.
- Cohen BJ, Beard S, Knowles WA et al. Chronic anemia due to parvovirus B19 infection in a bone marrow transplant patient after platelet transfusion // Transfusion. 1997; 37:947–952.
- Lamont RF, Sobel J, Vaisbuch E et al. Parvovirus B19 infection in human pregnancy // BJOG. 2011; 118(2):175–186.
- Van Beers-Tas MH, Heidema J. Review: Pathogenesis of parvovirus infections in children // Virol Mycol. 2013; 2 (1): 110.
- Anderson LJ Role of parvovirus B19 in human disease // Pediatr. Infect. Dis. 1987: 6: 711–718.
- Valentin MN, Cohen PJ Pediatric Parvovirus B19: spectrum of clinical manifestations // Cutis. 2013; 92: 179–184.
- Pavlovic MD Papular-purpuric “gloves and socks” syndrome caused by parvovirus B19 // Vojnosanit Pregl. 2003; 60(2):223–225.
- Heckler GT, Dal Ri NMK, Almeida HL Jr. Case for diagnosis // An Bras Dermatol. 2012; 87(5):793–794.
- Kumano K. Various clinical symptoms in human parvovirus B19 infection // Japanese Journal of Clinical Immunology. 2008; 31(6):448–453.
- Muir K., Todd WT, Watson WH, Fitzsimons E. Viral-associated haemophagocytosis with parvovirus-B19-related pancytopenia // Lancet. 1992; 339:1139–1140.
- Saarinen UM, Chorba TL, Tattersall P. et al. Human parvovirus B19-induced epidemic acute red cell aplasia in patients with hereditary hemolytic anemia // Blood. 1986; 67:1411–1417.
- Douvoyiannis M., Litman N., Goldman DL Neurologic manifestations associated with Parvovirus B19 Infection // CID. 2009; 48 (15): 1713–1723.
- Bihari Ch., Rastogi A., Saxena P. et al. Parvovirus B19 associated hepatitis // Hindawi Publishing Corporation // Hepatitis Research and Treatment. 2013; Article ID 472027: 1–9.
- Poole BD, Karetnyi YV, Naides SJ Parvovirus B19-induced apoptosis of hepatocytes // Journal of virology. 2004; 78(14):7775–7783.
- Hatakka A., Klein J., He R. et al. Acute hepatitis as a manifestation of parvovirus B19 infection // Journal of Clinical Microbiology. 2011; 49(9):3422–3424.
- Kindermann I., Barth C., Mahfoud F. et al. Update on Myocarditis // Journal of the American College of Cardiology. 2012; 59(9):779–792.
- Klingel K., Selinka H.-C., Sauter M. et al. Molecular mechanisms in enterovirus and parvovirus B19 associated myocarditis and inflammatory cardiomyopathy // European Heart Journal. 2002; 4 (1): 108–112.
- Schultheiss H.-P., Kuhl U., Cooper LT The management of myocarditis // European Heart Journal. 2011; doi:10.1093:1–13.
- Caforio ALP, Bottaro S., Iliceto S. Dilated cardiomyopathy (DCM) and myocarditis: classification, clinical and autoimmune features // Applied Cardiopulmonary Pathophysiology. 2012; 16:82–95.
- Santos Tavares P., Rocon-Albuquerque R., Leite-Moreira AF Innate immune receptor activation in viral myocarditis: pathophysiologic implications // Rev Port Cardiol. 2010; 29 (01): 57–78.
- Smith J. Human parvovirus B19: a literature review and case study // Infant. 2008; 4 (5): 101–104.
- Ergaz Z., Ornoy A. Parvovirus B19 in pregnancy // Reproductive Toxicology. 2006; 21: 421–435.
- Giorgio E., Antonietta De Oronzo M., Iozza I. Parvovirus B19 during pregnancy: a review // Journal of Prenatal Medicine. 2010; 4 (4): 63–66.
- Schulert GS, Walsh WF, Weitkamp JH Polymicrogyria and congenital Parvovirus B19 infection // Am J Perinatol Rep. 2011; 1: 105–110.
- Dieck D., Schild RL, Hansmann M., Eis-Hubinger AM Prenatal diagnosis of congenital parvovirus B19 infections: value of serological and PCR techniques in maternal and fetal serum // Prenatal Diagnosis. 1999; 19: 1119–1123.
- Bernstein DI, Sahly H., Keitel WA et al. Safety and immunogenicity of a candidate Parvovirus B19 vaccine // Vaccine. 2011; 29(43):7357–7363.
- Pickering LK, Baker CJ, Kimberlin DW et al. Parvovirus B19. Report of the Committee on Infectious Diseases // American Academy of Pediatrics. 2009: 491–494.
Yu. B. Belan1, Doctor of Medical Sciences, Professor M. V. Starikovich, Candidate of Medical Sciences
State Budgetary Educational Institution of Higher Professional Education Omsk State Medical Academy of the Ministry of Health of the Russian Federation, Omsk
1 Contact information
How can a child become infected with fifth disease?
Erythema infectiosum, like many other viral diseases, is transmitted by airborne droplets, that is, through the ingestion of respiratory secretion products (saliva, sputum, mucus) by virus carriers when sneezing or coughing. Infected people are most contagious when the symptoms of the disease are similar to those of a typical cold. Fifth disease is also transmitted through blood, so pregnant women are advised to avoid sick people, as the pathogen can be transmitted through the blood to the fetus.
What are the means to prevent fifth disease?
There is no vaccine for fifth disease. However, there are several recommendations that you, your family members and your children can follow to reduce the likelihood of infection in the family:
- Wash your hands frequently with soap and water for at least 20 seconds.
- Cover your nose and mouth when sneezing or coughing.
- Avoid touching your face, especially your nose, eyes and mouth.
- Try not to contact potentially infected people.
- If you have fifth disease, stay home and avoid contact with other people to avoid infecting them.
When should you contact a pediatrician?
If your child's condition worsens (for example, a sudden increase in temperature) or if his symptoms do not go away, contact your doctor as soon as possible. In children with weakened immune systems, erythema infectiosum is more severe. If your child has a weakened immune system, it is recommended to consult a pediatrician at the first symptoms of the disease.
With fifth disease, the baby may feel weak. To help him feel better in a few days or weeks, surround your child with care and love. But there is good news - the fifth disease goes away quite quickly, so everything will soon return to normal.
How this article was written The information presented in this article is based on expert advice published by trusted (medical and government) sources such as the American Pediatric Association and the American College of Obstetricians and Gynecologists. A complete list of links to sources used to write this article can be found at the end of the article. The information on this page is not a substitute for professional medical advice. Always consult your doctor for diagnosis and treatment.
Treatment
Regardless of whether viral erythema is detected in children or infectious, it is still worth following the doctor’s recommendations. Note that therapy, depending on the type of disease, is prescribed individually. The characteristics of the child’s body are also taken into account.
More often, treatment of erythema in children includes:
- taking antihistamines;
- taking vitamin complexes;
- treatment of local lesions with ointments, creams, aerosols;
- taking antibiotics (in certain cases).
Erythema nodosum, infectious and viral erythema in children is treated comprehensively. The child must be under the supervision of the attending physician. You can make an appointment with a highly qualified specialist in the center of Moscow by contacting JSC "Medicine" (clinic of Academician Roitberg), which is located at 2nd Tverskoy-Yamskaya lane 10 (not far from the Mayakovskaya, Belorusskaya, Novoslobodskaya, Tverskaya, Chekhovskaya metro stations) .