Article for the “bio/mol/text” competition: Doctors have long noticed that the occurrence of life-threatening exacerbations of cardiovascular diseases, such as myocardial infarction, stroke, serious attacks of arrhythmia, is often associated with a certain time of day - such complications appear much more often early morning. Having become interested in this feature, doctors conducted numerous studies and found that this phenomenon is closely related to the work of the body’s internal clock, and that when studying cardiovascular diseases, it is necessary to pay attention to the peculiarities of the regulation of the body’s circadian rhythms.
What is circadian rhythm
We live in accordance with the rhythms of nature: after night, day inevitably comes, darkness is necessarily replaced by light. And, in order to adapt to this regular alternation of conditions set by the external environment, our body has developed a very complex and as yet not yet fully understood mechanism of the internal clock - our “built-in chronometer,” which physiologists call the daily or circadian (circadian) rhythm. If literally translated from Latin, “circa” means about, and dia means “day”. That is, the circadian rhythm is a rhythm with a period of about a day. Why was this prefix “about” needed? The fact is that the time of completion of the full cycle of our “built-in chronometer” is still controversial among scientists, since the internal regularity of the body does not fit exactly into the 24 hours that make up our astronomical day.
In 1962, physiologist-researcher Aschoff, as an experiment, sent his sons to a sound- and light-proof bunker, where they lived, focusing only on their internal rhythms, and not on the alternation of light day and dark night. This study showed that the internal chronometers of regular variability in human physiological functions are actually tuned to a 25-hour rhythm [1]. But there is another opinion. For example, the results of an experiment led by the famous speleologist Michel Cifra demonstrated that participants imprisoned in a cave for several months experienced a gradual transition from 24-hour rhythms to 48-hour ones: a person needed 36 hours to be awake and 12 to sleep [2 ].
But, one way or another, there is no doubt that an internal biological clock operates in our body, and it works, as genetic research in recent years has revealed, in every cell of our body. The genetic nature of biological rhythms began to be revealed in 1971, when for the first time in the world the clock gene Per was found in the Drosophila fly - it was called an abbreviation for the word “period” [3]. It was observed that a mutation in this gene caused deviations in the periodicity of the circadian rhythm in flies. These studies laid the foundation for a number of discoveries, as a result of which the modern understanding of the molecular structure of the biological clock was formed.
Hierarchy of internal biological clocks
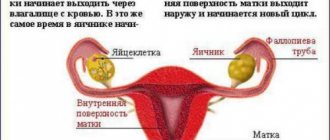
So, how does our internal clock work? Recent studies indicate that the internal pacemakers in our body are organized according to the laws of hierarchy: there are the most important clocks and subordinate clocks. The main center of the circadian clock is the suprachiasmatic nucleus in the brain, a dense cluster of about 20 thousand neurons, and it is located just next to the center that regulates the production of hormones in the body. As for the subordinate clocks, as analysis of gene expression in the cells of internal organs has shown, genes responsible for circadian rhythms are expressed in every cell of the body, including even connective tissue. This led scientists to believe that each organ has its own internal clock. The internal clock system of the internal organs was called the peripheral clock, and the suprachiasmatic nucleus that controls them was called the central clock (Fig. 1). The liver, blood vessels, heart, and kidneys have their own chronometer. But for the body to function effectively, it is extremely important that all clock mechanisms are tuned to work harmoniously in the same rhythm - synchronized.
Figure 1. Hierarchy of the internal biological clock: the main center of the circadian clock is the suprachiasmatic nucleus in the brain, which sets the rhythm of all cells of the body through the autonomic nervous system, specialized hormones and various factors. Subordinate clocks in the cells of internal organs are called peripheral.
The phases of internal chronometers can shift under the influence of certain stimuli that can impose their own rhythm. Such stimuli are called zeitgebers (from German Zeit - “time” and geben - “give”) or pacemakers. Each watch is capable of responding to its own specific pacemakers. For example, light sets the rhythm of the central clock in the suprachiasmatic nucleus, while it does not directly affect the peripheral clock. Zeitgebers can be not only external influences, but also behavioral patterns: physical activity regimen, sleep-wake cycle, and even diet. For example, it has been clearly shown that the internal clock of the liver is more tuned to the rhythm of food intake than to the rhythms of the light and dark periods of the day [4].
The main physiological synchronizer of all peripheral clocks is the suprachiasmatic nucleus. Thanks to their connections with the light-sensitive cells of the retina, the neurons of the suprachiasmatic nucleus are able to receive information about the photoperiod from the outside and adjust the internal rhythms of the body to external conditions. Synchronization of peripheral clock systems is carried out through the autonomic nervous system by special hormones and, possibly, other, as yet poorly understood, pathways. Every year, scientists discover and describe in detail more and more new factors that influence the regulation of internal rhythms [5].
How does it all work? A few words about the structure of the heart and its parts
The structure of the heart in mammals with 2 circles of blood circulation is approximately the same. The heart consists of two atria (the first chambers along the path of incoming blood), two ventricles, valves between these chambers, and vessels entering and leaving the heart with valves at their beginnings.
Between the right atrium and the right ventricle is the right atrioventricular
, or a
trioventricular
, which consists of 3 valves.
Therefore, it is called tricuspid
, or
tricuspid
.
Between the left atrium and the left ventricle is the left atrioventricular valve
, which consists of two valves and is called
mitral
.
Valves
, located at the mouths of the vessels extending from the heart, or the main vessels, namely
the aorta
and
pulmonary artery
, are called
“aortic”
and
“pulmonary”
.
Atrioventricular valves
– casement, i.e. their structure resembles doors on the wings: opened and closed, down - up.
Aortic valves
and
the pulmonary artery are different in structure
. Each of them consists of 3 semilunar valves that close in the center. When they open, they press against the wall of their vessel (aorta or pulmonary artery), and close, completely closing the lumen of the vessel. Moreover, their appearance resembles a brand name.
The tissue of the valves themselves, both atrioventricular and semilunar, is thin, even transparent in children, but amazingly elastic and durable, designed by nature for continuous rhythmic work, amounting to billions of monotonous actions.
Between the cavities of the heart, or its chambers, there are partitions that separate the flow of venous and arterial blood. This is the interatrial septum, i.e. between the right and left atria, and the interventricular septum - between the right and left ventricles. In a normal, formed heart, they are completely closed, there are no holes or defects in them
and thus blood never flows from one half of the heart to the other.
Let us dwell in more detail on the anatomical structure of the heart and its chambers. After all, even those that have the same name (atria or ventricles) are structured completely differently and perform different functions.
The shape of the heart resembles a pear lying somewhat on its side, with the top located to the left and below, and the base to the right and above. The apex of the heart is the part of the heart whose movements can be felt if you place your palm on the chest in the fifth intercostal space to the left of the sternum. You can easily feel its push both in yourself and in your child. These are the movements of the apex of the heart with each contraction. The contractions are almost synchronous with the pulse, which can also be easily felt on the arm (where the forearm meets the hand) or on the cervical vessels. The pulse is the filling of the vessels with a wave of blood coming from the heart with each contraction. Pulse frequency and its rhythm are an indirect and easily accessible reflection of the activity of the heart itself.
The apex is the most mobile part of the heart, although the whole thing, all its departments, are in constant motion.
The work of the heart, its movement, consists of two alternating phases - contraction (systole) and relaxation (diastole).
The rhythmic, constant alternation of these phases, necessary for normal functioning, is ensured by the occurrence and conduction of an electrical impulse through a system of special cells - through the nodes and fibers of the conduction system of the heart
.
Impulses arise first in the uppermost, so-called sinus node
, then pass to the second,
atrioventricular node
, and from it - along thinner fibers - to the muscles of the right and left ventricles, causing contraction of all their muscles.
Right atrium
accepts venous blood from the vena cava, i.e. from the whole body and in addition the venous blood of the heart itself. This is the largest chamber in volume and, perhaps, the most stretchable chamber of the heart. If necessary, it can accommodate several times more blood than under normal conditions, i.e. has a gigantic “reserve” of volume. The wall of the right atrium consists of a layer of thin muscle fibers. In addition to the function of “receiving” venous blood, the right atrium performs the function of a cardiac pacemaker. Both main nodes of the conduction system of the heart lie in its walls.
The right atrium connects or, more precisely, opens into the right ventricle through the atrioventricular foramen
regulated
by the tricuspid valve
. This hole is wide enough to allow the entire volume of blood to pass from the atrium into the right ventricle during the period of relaxation of its muscles, i.e. in the diastole phase, and fill its cavity.
The right ventricle is a much thicker-walled muscular structure than the atrium. This is the most anterior part of the heart, lying immediately under the sternum. It is relatively stretchable when needed. The shape of its cavity resembles a new moon appearing in the sky. If you look closely, you can see how the luminous stripe of the month in a semicircle covers a large dark ball of the unlit part of the Moon. Likewise, the right ventricle flows around the powerful cylindrical left ventricle with its cavity.
Inside, this ventricle consists of two cones that extend into each other: the inlet cone and the outlet cone. They converge at their apices at the apex of the heart and are separated at the top by a muscular ridge, the so-called supraventricular crest.
The right ventricle opens into the pulmonary artery, which, together with the aorta, is the so-called main, or “great” vessel. At the transition from the ventricle to the pulmonary artery there is a tricuspid, semilunar valve of the pulmonary trunk, which allows blood to pass in one direction - to the lungs.
The left atrium is the most posterior of the cardiac chambers. It receives oxygenated, arterial blood from the pulmonary veins. There are only four veins and they flow into the posterior wall of the left atrium. The chamber of this atrium is much smaller than the right one, and its ability to stretch is significantly less.
The left atrium opens through the atrioventricular orifice into the left ventricle. This hole contains a bicuspid - mitral - valve, the opening and closing of which regulates the process of filling and emptying of the ventricle in the systole and diastole phases.
The left ventricle is the main one in the heart, and in the entire circulatory system. This is a powerful muscular chamber, the walls of which are 3-4 times thicker than those of its right neighbor. It is a compact cone with the inlet (with the mitral valve) and outlet (with the tricuspid aortic semilunar valve) lying next to each other and closely interconnected.
In order for this entire complex system to work harmoniously and efficiently, it must receive constant necessary nutrition in the form of oxygen and nutrients, and waste products must be removed. For this purpose, there are arterial and venous systems of the heart itself.
The arterial system of the heart itself consists of two - the left
and
the right
-
coronary (coronary) arteries
, which depart at the very beginning, at the mouth of the ascending aorta. These are its first branches. They immediately divide into smaller ones and distribute blood to all parts of the continuously moving heart. The “spent” blood, which has given up oxygen, flows through numerous small veins, which gather into one large one - the coronary sinus - and flow into the cavity of the right atrium. Thus, the heart feeds itself, and its function directly depends on the correct position and condition of the coronary arteries.
So, let's summarize. Anatomically, the heart is a powerful muscular organ with four chambers and four valves. The structure of the chambers and valves is different from each other, because subordinated to performing various tasks. The right parts of the heart are separated from the left by partitions and do not communicate with each other.
Quoted from the book by G. E. Falkovsky, S. M. Krupyanko. The heart of a child. A book for parents about congenital heart defects
How to get treatment at the Scientific Center named after. A.N. Bakuleva?
Online consultations
Loss of synchronization and disease progression
As experiments show, synchronization of all internal rhythms is an extremely important condition for maintaining health and life expectancy. When scientists study the relationship between a malfunctioning biological clock and heart disease, the obvious question that arises is what comes first: a breakdown in the internal clock causes heart disease, or is it the heart pathology itself that causes the malfunction of our built-in chronometers? In an attempt to answer this question, at least two opposing hypotheses have been put forward.
In support of the hypothesis that the loss of synchronization of internal rhythms is primary in the onset of the disease, a number of interesting experiments were conducted. Researcher Tami Martino analyzed the lifespan of golden hamsters with a specific mutation in the tau gene, which reduces the period of the circadian rhythm in the peripheral clock to 22 hours (Figure 2). In other words, the internal clock of this line of cheeky rodents is very fast. It turned out that the overall life expectancy of hamsters with the mutation is reduced by 20%, and they die at an early age from serious myocardial diseases - fibrosis and cardiomyopathies [6].
Figure 2. Golden hamster with a mutation in the tau gene: the hamster's internal clock is fast by two hours a day. The lack of synchronization of internal and external rhythms led to the fact that the rodent developed serious health problems - myocardial hypertrophy.
However, when artificial conditions were created for such hamsters so that the period of alternating light and darkness was 22 hours, the cardiac pathology was replaced by normal functioning of the heart. Moreover, removal of the suprachiasmatic nucleus, the main clock of the body, also had a preventive effect: myocardial hypertrophy did not develop in golden hamsters after the operation. What is the reason for such a miraculous healing?
The results obtained indicate that it is not so much damage to the peripheral clock as loss of synchronization between the central and peripheral pacemakers that leads to the occurrence of cardiovascular pathology. In mutant hamsters, there was a mismatch between the 22-hour period of the peripheral clock and the 24-hour period of the central clock. When a rhythm of 22 o'clock was imposed on the central chronometer through changes in external conditions (light/dark), it was synchronized with the peripheral clock, and cardiac pathology did not develop. And when the suprachiasmatic nucleus was removed, then again nothing prevented the peripheral clock from freely implementing its own rhythm, and the hamster’s heart was again saved.
On the other hand, the disease itself can disrupt the coherence of internal biorhythms. For example, during acute myocardial infarction, damaged cells undergo a phase shift in the circadian clock relative to healthy tissues. This loss of synchronization is very dangerous and can cause life-threatening arrhythmia attacks.
Restoring the coherence of heart cell rhythms with the natural cycles of other organs and tissues and with cyclical changes in environmental conditions may be a promising strategy in the fight against cardiovascular diseases. But to implement this direction, very deep knowledge about the patterns of functioning of biorhythms is required. Interestingly, even in healthy people, the circadian rhythm of the cells in the inner lining of the veins varies depending on their anatomical location. Further research is needed to identify all the zeitgebers in the body as accurately as possible, and to use this knowledge to repair our built-in chronometers if they fail.
How does the heart develop (form)?
To form all body systems, the fetus requires its own blood circulation. Therefore, the heart is the first functional organ to appear in the body of a human embryo; this occurs approximately in the third week of fetal development.
At the very beginning, an embryo is just a collection of cells. But as pregnancy progresses, there are more and more of them, and so they connect, forming into programmed forms. First, two tubes are formed, which then merge into one. This tube folds and rushes down to form a loop - the primary cardiac loop. This loop outstrips all other cells in growth and quickly lengthens, then lies to the right (maybe to the left, which means the heart will be positioned mirror-like) in the form of a ring.
So, usually on the 22nd day after conception, the first contraction of the heart occurs, and by the 26th day the fetus has its own blood circulation. Further development involves the appearance of septa, the formation of valves and remodeling of the heart chambers. The septums are formed by the fifth week, and the heart valves will be formed by the ninth week.
Interestingly, the fetal heart begins to beat at the normal rate of an adult - 75-80 beats per minute. Then, by the beginning of the seventh week, the pulse is about 165-185 beats per minute, which is the maximum value, and then a slowdown follows. The newborn's pulse is within the range of 120-170 beats per minute.
Daily variability of cardiovascular parameters
Another very important point is that during the day the heart’s sensitivity to stress, emotional and physical stress varies. The indicators of cardiovascular function themselves also change over time: blood pressure, blood flow speed, heart rate and others. Continuous recording of an electrocardiogram for 24 hours in people at rest shows that a person's heart rate is constantly varying: it reaches a minimum in the fifth or sixth hour of sleep and at this time is 48–50 beats per minute. It reaches its maximum in the evening, at about 6 p.m., and then gradually begins to decrease again.
All these phenomena are possible due to the complex molecular mechanisms of the cardiovascular system's own peripheral clock. About 10% of genes expressed in cardiac cells have a daily rhythm of expression. Currently, an active search is being carried out for factors affecting the functioning of the heart and having circadian rhythm. Molecular clocks have already been found in the muscle cells of the heart, in the cells of the inner lining of blood vessels (in the endothelium) and in the muscle cells of blood vessels.
How does the human heart work?
Different peoples view the human heart as the seat of romantic feelings, mind or soul. It has great significance in many cultures and has attracted attention since ancient times.
First of all, the heart is interesting because its shape and size depend on the age, gender, build and state of health of each person. Figuratively, the organ is usually compared to a medium-sized fist weighing about 500 g. These indicators vary widely, but in any case, the human heart looks completely different from what we are used to seeing on valentines and cards. If you want to know what your heart looks like, we recommend that you consult a doctor and have an ultrasound scan of the organ. In this way, it will also be possible to identify possible pathologies of the cardiovascular system.
How many chambers are there in the heart and how is it structured? Modern anatomy of the human heart has revealed all the secrets and, first of all, scientists have studied the structure of the heart. It was briefly described beautifully, for example, by the authors Roen Johannes V., Yokochi C. and Lutjen-Drekoll E. in the Great Atlas of Anatomy. It provides colorful and vivid answers to the following questions: how many chambers does the human heart have and how many valves are there in the human heart, what are the arteries and veins of the heart.
Photo: Reneva N.B., Sonin N.I. Biology. Human. 8th grade. Methodological guide to the textbook by N.I. Sonin, M.R. Sapin “Biology. Human. 8th grade". – M.: Bustard, 2001. – P.46–49.: UGC
The structure of the human heart is as follows:
- There are four chambers of the heart. The muscular septum divides the organ cavity into two halves, each of which is further divided in half;
- the upper parts of the heart are called atria, the lower parts are called ventricles;
- all chambers and the blood vessels with which they communicate are separated by valves.
Heart valves are necessary for blood flow in one direction and have the following names:
- the right atrium and the right ventricle of the heart are separated by the tricuspid valve;
- the left atrium and left ventricle are separated by the bicuspid mitral valve;
- between the right ventricle and the pulmonary artery is the pulmonary valve;
- The left ventricle borders the aorta via the aortic valve.
The two coronary arteries supply blood to the heart itself. Their structure also includes valves to prevent reverse blood flow. In addition, the organ has so-called pacemakers, whose task is to produce impulses and control the contraction and relaxation of the muscle.
Molecular clock in cardiac muscle cells
Recently, scientists published a report in the journal Nature that the protein Klf 15 (kruppel-like factor), which controls the processes of tissue formation, fat metabolism and inflammation in the body, can also influence the circadian rhythms of the heart. The concentration of this protein varies depending on the stage of the sleep-wake cycle. The researchers bred lines of mice with two variant mutations in the gene encoding Klf 15, which led to the fact that the level of the factor in the blood plasma was either excessively elevated or the protein was absent altogether. In both cases, the mice suffered from life-threatening cardiac arrhythmias [7].
Upon deeper study, it turned out that Klf 15 is only the first step in a complex molecular cascade, because it controls another protein, KChIP 2 (Kv channel-interacting protein), a factor that interacts with potassium channels in cardiac muscle cells. Changes in the concentration of KChIP 2 lead to electrical instability of heart tissue and, as a consequence, to cardiac arrhythmias; Moreover, the gene for this factor has a daily rhythm of expression.
The potassium channel genes of cardiac muscle cells Kv1.5 and Kv4.2 also have a daily rhythm of expression. Interestingly, the expression of Kv1.5 increases during the dark period, while messenger RNA of the Kv4.2 protein is found in higher concentrations during the light period. Rhythm disturbances in any link of this complex system can be associated with the daily time of occurrence of arrhythmia attacks.
Functions of the human heart
The heart works tirelessly to ensure that blood moves through the vessels, is enriched with oxygen in the lungs and delivers it to every cell of the body. This function of the heart is rightfully considered the main one and, for simplicity, is called pumping.
For the correct implementation of this task, the following properties of the heart muscle, which are also known as the basic functions of the heart, are important:
Automatic
This concept hides the ability for rhythmic contractions, thanks to electrical impulses produced by the heart itself. Among the muscle cells of the organ there are specific areas that are endowed with this quality.
They are also called pacemakers. The main node is located in the area of the right atrium. It is he who sets the tone for the heart and determines the frequency of contractions. Changes in the body can affect the work of the pacemaker, but normally it works autonomously.
Excitability
After the pacemaker has generated an impulse, it must instantly spread throughout the heart. Only in this case will the contraction cover the entire atrium or ventricle. This is possible due to the high susceptibility of heart cells to impulses, as well as the many contacts between them.
In simpler terms, we can say that the heart muscle is very sensitive, and its cells are a very close-knit group.
Conductivity
To respond to an impulse as quickly as possible, special pathways are provided in the heart. Through this system, signal transmission occurs instantly, reaching the most remote areas.
By the way, the electrocardiograph records precisely the moments of impact of impulses on all cardiac chambers.
Contractility
The length of muscle fibers and their elasticity give the heart the ability to contract effectively and work without days off or vacation. The force of contraction is necessary to push blood in the desired direction.
Refractoriness
After each contraction, relaxation occurs in the heart. It lasts a fraction of a second, but allows the cells to return to their original position and is the key to the same heart rhythm that we feel when we put our hand to our chest.
Synchronization of the molecular clock of cardiac muscle cells with lipid metabolism
We have already talked about how important it is to synchronize heart rhythms with the cycles of other physiological systems of the body. It is equally important to note that some internal cycles are capable of imposing their rhythm on the heart clock. One of these master cycles is the daily rhythm of the circulation of fatty acids and lipid levels, which is tightly linked to the circadian one. Fatty acids are the primary “heart fuel”: 70% of them are utilized by the heart. With an excess of fatty acids, the contractile function of the heart is suppressed, and the heart responds to these changes in the internal environment by activating both oxidative (mitochondrial) and non-oxidative metabolism. In this way, the heart reduces cellular toxicity caused by fatty acid loading. And this process is also associated with the daily rhythms of gene expression.
American researcher Molly Bray studied circadian clock genes using DNA microarray technology. She was able to identify 548 genes that regulate the clock in atrial cardiomyocytes and 176 genes associated with the circadian rhythm of ventricular muscle cells. These included genes involved in lipogenesis and lipid binding proteins; all of them showed diurnal expression [8].
Circulatory system
Circulatory system (animation)
The human cardiovascular system is formed by two circles of blood circulation. With each heartbeat, blood moves in both circles at once.
Pulmonary circulation
- Deoxygenated blood from the superior and inferior vena cava enters the right atrium and then into the right ventricle.
- From the right ventricle, blood is pushed into the pulmonary trunk. The pulmonary arteries carry blood directly to the lungs (to the pulmonary capillaries), where it receives oxygen and releases carbon dioxide.
- Having received enough oxygen, the blood returns to the left atrium of the heart through the pulmonary veins.
Systemic circulation
- From the left atrium, blood moves into the left ventricle, from where it is subsequently pumped through the aorta into the systemic circulation.
- After going through a difficult path, the blood again arrives through the vena cava to the right atrium of the heart.
Normally, the amount of blood pushed out of the ventricles of the heart is the same with each contraction. Thus, an equal volume of blood simultaneously enters the greater and lesser circulation.
What is the difference between veins and arteries?
- Veins are designed to transport blood to the heart, and the job of arteries is to supply blood in the opposite direction.
- In veins, blood pressure is lower than in arteries. Accordingly, the walls of arteries are more elastic and dense.
- Arteries saturate “fresh” tissue, and veins take away “waste” blood.
- In the case of vascular damage, arterial or venous bleeding can be distinguished by its intensity and the color of the blood. Arterial - strong, pulsating, beating like a “fountain”, the color of the blood is bright. Venous - bleeding of constant intensity (continuous flow), the color of the blood is dark.
Peripheral clock in endothelial cells
Several groups of scientists have demonstrated the role of clock genes in the function of the endothelium, the tissue lining the inside of blood vessels and the heart. They found that in mice with a mutation in the Per 2 clock gene, blood vessels do not relax in response to the influence of the main relaxing neurotransmitter, acetylcholine. In addition to this very unpleasant dysfunction, a very high concentration of substances that stimulate vascular compression is detected in the blood of mice, which is fraught with the occurrence of arterial hypertension [9].
But the health problems for the unfortunate mice did not end there. Researcher Chao Wang showed that if endothelial cells have a mutation of the Per 2 gene, then blood vessels quickly age, are poorly restored after damage, and the life expectancy of rodents themselves is greatly reduced [10].
Peripheral clocks in vascular muscle cells
The smooth muscle cells of blood vessels - myocytes - also have their own peripheral clock. Takishige Kunieda studied the circadian system in aging vascular myocytes. He found that in these cells, the loss of circadian rhythmicity is associated with telomere shortening. The introduction of telomerase prevented problems with the expression of clock genes. These studies suggest that telomerase regulation may be one of the therapeutic options for age-related circadian rhythm disorders [11].