What is Motilium
The active component of the drug is domperidone, a substance that can quickly suppress nausea, normalize peristalsis, stool, and relieve signs of indigestion. The product is produced in several pharmaceutical forms:
- film-coated tablets: white, biconvex, containing 10 mg of domperidone, packaged in blisters;
- non-coated sublingual tablets with mint flavor;
- syrup for children: a sweetish, thick, white, water-based liquid with a dosage of domperidone 1 mg per 1 ml, sold in glass bottles with a measuring syringe.
The tablet medicine contains auxiliary compounds: starch, povidone, stabilizers.
Motilium®
Anticholinergic drugs can neutralize the effect of MOTILIUM. The oral bioavailability of MOTILIUM decreases after previous administration of cimetidine or sodium bicarbonate. You should not take antacid and antisecretory drugs simultaneously with MOTILIUM, as they reduce its bioavailability after oral administration (see section “Special Instructions”).
The main role in the metabolism of domperidone is played by the CYP3A4 isoenzyme. In vitro studies and clinical experience indicate that concomitant use of drugs that significantly inhibit this isoenzyme may cause increased plasma concentrations of domperidone. Strong CYP3A4 inhibitors include:
— Azole antifungals, such as fluconazole*, itraconazole, ketoconazole* and voriconazole*;
— Macrolide antibiotics, for example clarithromycin* and erythromycin*;
- HIV protease inhibitors, such as amprenavir, atazanavir, fosamprenavir, indinavir, nelfinavir, ritonavir and saquinavir;
- Calcium antagonists, such as diltiazem and verapamil;
— Amiodarone*;
- Aprepitant;
- Nefazodone.
(Drugs marked with an asterisk also prolong the QTc interval (see section “Contraindications”)).
In a number of studies of the pharmacokinetic and pharmacodynamic interactions of domperidone with oral ketoconazole and oral erythromycin in healthy volunteers, these drugs have been shown to significantly inhibit the primary metabolism of domperidone by the CYP3A4 isoenzyme.
With simultaneous administration of 10 mg of domperidone 4 times a day and 200 mg of ketoconazole 2 times a day, there was an increase in the QTc interval by an average of 9.8 ms during the entire observation period, at certain points the changes varied from 1.2 to 17.5 ms. With simultaneous administration of 10 mg of domperidone 4 times a day and 500 mg of erythromycin 3 times a day, there was an increase in the QTc interval by an average of 9.9 ms during the entire observation period, at certain points the changes varied from 1.6 to 14.3 ms. In each of these studies, the Cmax and AUC of domperidone were increased approximately threefold (see section "Contraindications").
It is currently unknown how elevated plasma concentrations of domperidone contribute to changes in the QTc interval.
In these studies, domperidone monotherapy (10 mg four times daily) prolonged the QTc interval by 1.6 ms (ketoconazole study) and 2.5 ms (erythromycin study), whereas ketoconazole monotherapy (200 mg twice daily) and erythromycin (500 mg three times daily) led to a prolongation of the QTc interval by 3.8 and 4.9 ms, respectively, during the entire observation period.
In another multiple-dose study in healthy volunteers, there was no significant prolongation of the QTc interval during inpatient domperidone monotherapy (40 mg four times daily, total daily dose 160 mg, 2 times the recommended maximum daily dose). However, plasma concentrations of domperidone were similar to those in studies of the interaction of domperidone with other drugs.
Theoretically, since MOTILIUM® has a gastrokinetic effect, it could influence the absorption of concomitantly administered oral drugs, in particular sustained-release or enteric-coated drugs. However, the use of domperidone in patients taking paracetamol or digoxin did not affect the blood levels of these drugs.
MOTILIUM® can be taken simultaneously with:
- neuroleptics, the effect of which it does not enhance;
- with dopaminergic receptor agonists (bromocriptine, L-dopa), since it inhibits their unwanted peripheral effects, such as digestive disorders, nausea and vomiting, without affecting their central effects.
For what diseases is Motilium needed?
The medicine helps restore well-being in the following pathologies:
- stomach ulcer;
- atonic bowel dysfunction;
- flatulence;
- hypokinetic dyskinesia of the gallbladder;
- epigastric pain;
- nausea, vomiting due to food or chemical intoxication;
- digestive disorders due to taking medications, radiation therapy;
- spontaneous constipation;
- frequent regurgitation in children.
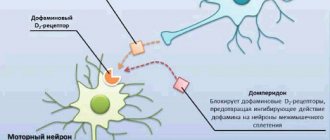
The drug moderately stimulates intestinal peristalsis, normalizing gastric functions and eliminating signs of dyspepsia. Domperidone suppresses dopamine activity, producing a mild antipsychotic effect. But, unlike other drugs, it does not lead to autonomic disorders.
The medicine quickly penetrates the blood through the mucous membranes and interacts with its proteins. After administration, Motilium cleanses the stomach cavity, intestinal lumen, binds and neutralizes toxic substances. It is completely excreted from the body with intestinal contents after 12–24 hours, without accumulating in tissues.
MOTILIUM suspension for oral administration 1 mg/ml bottle 100 ml
Motilium is an antiemetic. Pharmacodynamics Domperidone is a dopamine antagonist that, like metoclopramide and some antipsychotics, has antiemetic properties. However, unlike these drugs, domperidone does not penetrate the blood-brain barrier well. The use of domperidone is rarely accompanied by extrapyramidal side effects, especially in adults, but domperidone stimulates the release of prolactin from the pituitary gland. Its antiemetic effect may be due to a combination of peripheral (gastrokinetic) action and antagonism of dopamine receptors in the chemoreceptor trigger zone. When administered orally, domperidone increases the duration of antral and duodenal contractions, accelerates gastric emptying and increases lower esophageal sphincter pressure in healthy people. Domperidone has no effect on gastric secretion. Pharmacokinetics Domperidone is rapidly absorbed when administered orally on an empty stomach, with peak plasma concentrations occurring within approximately 1 hour. The low absolute bioavailability of oral domperidone (approximately 15%) is due to extensive first-pass metabolism in the intestinal wall and liver. Domperidone should be taken 15-30 minutes before meals. Hypoacidity of gastric juice reduces the absorption of domperidone. When taking the drug after a meal, it takes longer to achieve maximum absorption and the area under the curve (AUC) increases slightly. When taken orally, domperidone does not accumulate or induce its own metabolism. The maximum plasma concentration 90 minutes after oral administration at a dose of 30 mg per day for 2 weeks was 21 ng/ml, almost no different from the maximum concentration after a single dose (18 ng/ml). Domperidone is 91-93% bound to plasma proteins. Concentrations of domperidone in breast milk of lactating women are 4 times lower than the corresponding concentrations in plasma. The drug is metabolized in the liver by hydroxylation and N-dealkylation. Excretion in urine and feces accounts for 31 and 66% of the oral dose, respectively. The excretion of the drug unchanged is a small percentage (10% in feces and approximately 1% in urine). The plasma half-life after taking a single dose is 7-9 hours in healthy people, but is prolonged in patients with severe renal failure (20.8 hours).
How to take Motilium tablets
Adults are recommended to take 1 pill three times a day, 15–20 minutes before meals. The coated tablets are washed down with cool or warm water. Sublingual - placed under the tongue until completely dissolved. If necessary, the single dose is increased 2–3 times. The maximum number of tablets per day is 8 pcs. It is allowed to take the medicine before bedtime.
For children, treatment with Motilium in tablets is recommended from 6–7 years of age: 1 tablet 2–3 times a day. For body weights less than 35 kg, it is recommended to use a suspension of the drug.
Motilium, 1 piece, 100 ml, 1 mg/ml, oral suspension
Domperidone is rapidly absorbed after oral administration on an empty stomach, the maximum plasma concentration (Cmax) is reached within 30-60 minutes. The low absolute bioavailability of domperidone when taken orally (approximately 15%) is associated with extensive first-pass metabolism in the intestinal wall and liver. Despite the fact that the bioavailability of domperidone in healthy people increases when taking the drug after meals, patients with complaints from the gastrointestinal tract (GIT) should take domperidone 15-30 minutes before meals. A decrease in gastric acidity leads to a decrease in the absorption of domperidone. Oral bioavailability is reduced by prior administration of cimetidine and sodium bicarbonate. When taking the drug after a meal, it takes longer to achieve maximum absorption and the area under the active substance concentration-time curve (AUC) increases slightly.
When taken orally, domperidone does not accumulate and does not induce its own metabolism; the maximum plasma concentration of 21 ng/ml at 90 minutes after 2 weeks of oral administration of the drug at a dose of 30 mg per day was almost the same as the maximum plasma concentration of 18 ng/ml after taking the first dose. Domperidone is 91-93% bound to plasma proteins. Distribution studies in animals using a radiolabeled drug have shown significant tissue distribution but low concentrations in the brain. Small amounts of the drug cross the placenta in rats.
Domperidone undergoes rapid and extensive metabolism in the liver by hydroxylation and N-dealkylation. In vitro metabolism studies using diagnostic inhibitors have shown that CYP3A4 is the main form of cytochrome P450 involved in the N-dealkylation of domperidone, while CYP3A4, CYP1A2 and CYP2E1 are involved in the aromatic hydroxylation of domperidone. Excretion in urine and feces is 31% and 66% of the oral dose, respectively. The proportion of the drug excreted unchanged is small (10% in feces and approximately 1% in urine). The plasma half-life after a single oral dose is 7-9 hours in healthy people, but increases in patients with severe renal impairment. In such patients (serum creatinine level >6 mg/100 ml, i.e. >0.6 mmol/L), the half-life of domperidone increases from 7.4 to 20.8 hours, but plasma concentrations of the drug are lower than in people with normal kidney function. A small amount of unchanged drug (about 1%) is excreted by the kidneys.
In patients with moderate hepatic impairment (Pugh score 7-9, Child-Pugh class B), the AUC and Cmax of domperidone are 2.9 and 1.5 times higher than in healthy individuals, respectively. The unbound fraction increases by 25% and the terminal half-life increases from 15 to 23 hours. In patients with mild hepatic impairment, systemic exposure is slightly reduced compared to healthy controls based on Cmax and AUC values, with no change in protein binding or terminal half-life. No data are available for patients with severe hepatic impairment. Pharmacokinetic data for children are not available.
Side effects of Motilium
The drug increases the synthesis of the hormone prolactin. In some cases, this can cause a short-term disruption of the menstrual cycle and provoke the development of gynemastia. Occasionally during treatment the following are possible:
- decreased appetite;
- dryness or unpleasant taste in the mouth;
- increased drowsiness;
- increased spasms in the intestines.
If any undesirable symptoms occur, you should consult your doctor.
MOTILIUM (suspension)
kov doctors and 3 from the emergency room.
On the 5th day, the child, who usually runs around with a temperature of 39.3 as if healthy, lay down with a cloth on the sofa. Then I decided to act tough. I quickly packed the bag for the hospital and called an ambulance. We were offered a Cerucal injection. Glory to this medicine! I finally achieved that the child began to retain fluid. On this day, my mother, a doctor, came to us from another city. She said: there is no point in sitting at home outside, it’s spring, we need to go for a walk. We went for a walk and walked about 1.5 km. My child trained 8 km. passes healthy, as if there is nothing to do. We came home. The child sat on the potty (sorry for the details), but about 0.6 liters of sour cream-like feces came out of him. Then I realized that the child had an obstruction. If it had taken another 1-2 days it would have been impossible to save him. My child has 2 terrible characteristics: he does not like to drink and poop (he always tries to restrain the urge so as not to be distracted from an interesting activity). This causes another problem: constipation and intoxication with one’s own feces. At the end of October, everything repeats itself. I have already told the doctors the story that happened in the spring. Doctors again do not undertake to diagnose anything. The child (4.5 years old) is still an artist, sometimes he lies down and rolls his eyes, sometimes he walks and staggers. That’s when we were prescribed Motilium along with Cerucal. We take it for three days. The child gradually began to recover. I began to eat and retain at least a quarter of my usual daily diet. Suddenly, on the evening of the third day, the child immediately began not just screaming, but screaming louder than women in labor during their last contraction, rushing from corner to corner, and covered in sweat. To all questions, he only answers: his butt hurts. In general, since he learned to speak from me, he knows that crying and screaming will not achieve anything. It is clear that the child is experiencing inhuman torment. I called an ambulance and quickly threw on a bag, preparing to be hospitalized. At the moment when the ambulance doctors arrived, the child suddenly rushes headlong onto the potty. The stool is not bulky, but smelly. Thanks to this, the emergency doctors were able to understand that it was an intestinal infection. When they found out that I was giving the child Motilium, they were so indignant (they just didn’t shoot me). They said that Motilium should not be prescribed for vomiting at all. They explained that if a child vomits, then the process of digestion does not come to pass, and improving digestion and intestinal movement on an empty stomach is a crime. Which leads to a very painful and often false (because there is simply nothing to do with it) urge to defecate. They said that they are repeatedly induced with such symptoms after Motilium against the background of vomiting.
After reading the instructions I was surprised. The instructions include indications for use in case of vomiting. Motilium was canceled for us. The next day we fought our way to the gastroenterologist without an appointment, arguing that we had an emergency rather than a chronic condition. The doctor confirmed the words of his ambulance colleagues and said that in our case Motilium should not be prescribed. Prescribed adequate treatment. After 2 days, the child was almost healthy.
To my question: what about all the indications in the instructions, he said that they were written in a streamlined manner and not correctly. And those who prescribe Motilium for vomiting and intestinal infections are not competent doctors. Motilium is intended to improve digestion and for frequent constipation.
I would like to note the miracle effect of Motilium. Despite the fact that the child took it for only three days, there have been no problems with bowel movements or constipation for a month now. Everything is like clockwork at the same time. The child developed a voracious appetite. He eats whatever you give him. Thanks to such an appetite, I finally taught him to eat salads from fresh vegetables every day. The child didn’t really like fruit either, and now he asks for an apple. Of course, the price of the product is not small; it cost us 721 rubles. If we had been prescribed it a couple of years ago, when problems with constipation had just begun, I think now my child would have a stronger immune system, since he would have an appetite and eat healthy foods, and would not poison himself with his own feces.
Therefore, I consider Motilium a very effective drug, especially if it is prescribed correctly.
When is Motilium contraindicated?
It is prohibited to use the medicine:
- in case of danger of perforation of a gastric ulcer, development of internal bleeding;
- in case of intestinal obstruction;
- in case of dysfunction, neoplasms in the pituitary gland;
- prolactinoma;
- severe kidney or liver damage;
- individual intolerance to any of the components of the drug.
If you are allergic to Motilium, itching, hyperemia, rash, lacrimation, and swelling of the mucous membranes are likely to occur. In such cases, discontinuation of therapy and replacement of the drug with another is required.
How does Motilium interact with other drugs?
During the period of using Motilium, it is advisable to stop using antacids and proton pump inhibitors - drugs that reduce the acidity of gastric juice. Simultaneous use slows down the action of Motilium and reduces its absorption.
Antimycotic drugs and drugs from the macrolide group inhibit the metabolism of domperidone. This is important to consider in cases of impaired renal and liver function.
When undergoing treatment with Ketoconazole, Azithromycin, Erythromycin and inhibitors of the CYP3A4 isoenzyme, Motilium should be discontinued. Otherwise, the risk of heart failure increases.
Motilium suspension for oral administration 1 mg/ml 100 ml bottle 1 pc. in Moscow
When using Motilium® together with antacid or antisecretory drugs, the latter should be taken after meals and not before meals, i.e. they should not be taken simultaneously with Motilium®
Use in children
Motilium® in rare cases may cause neurological side effects (see section "Side effects"). In this regard, the recommended dose should be strictly adhered to (see section “Method of administration and dosage”). Neurological undesirable effects can be caused by an overdose of the drug in adolescents, but other possible causes of such effects must be taken into account.
Use for diseases of the cardiovascular system.
Some epidemiological studies have suggested that domperidone may be associated with an increased risk of serious ventricular arrhythmias or sudden coronary death.
(see section "Side effects"). The risk may be more likely in patients over 60 years of age and in patients taking the drug in daily doses of more than 30 mg.
Patients over 60 years of age should take Motilium® EXPRESS with caution. The use of domperidone and other drugs that lead to prolongation of the QTc interval is not recommended in patients with existing conduction disorders, in particular, prolongation of the QTc interval, and in patients with severe electrolyte imbalance (hypokalemia, hyperkalemia, hypomagnesemia) or with bradycardia, or in patients with concomitant heart diseases such as congestive heart failure. As is known, against the background of electrolyte imbalance and bradycardia, the risk of arrhythmias increases.
If signs or symptoms appear that may be associated with cardiac arrhythmia, Motilium® therapy should be discontinued and a physician should be consulted.
Use for kidney diseases.
Since a very small percentage of the drug is excreted unchanged by the kidneys, single dose adjustment is not required in patients with renal failure. However, when re-prescribing Motilium®, the frequency of use should be reduced to one or two times a day, depending on the severity of renal dysfunction (see section "Method of administration and dosage"). During long-term therapy, patients should be monitored regularly.
Potential for drug interactions
The main route of metabolism of domperidone is through the CYP3A4 isoenzyme.
In vitro
Data and results from human studies indicate that concomitant use of drugs that significantly inhibit this enzyme may be associated with increased plasma concentrations of domperidone. The combined use of domperidone with potent inhibitors of the CYP3A4 isoenzyme, which, according to data obtained, cause prolongation of the QT interval, is contraindicated (see section “Contraindications”).
Caution is advised when co-administering domperidone with potent CYP3A4 inhibitors that do not prolong the QT interval, such as indinavir, and patients should be closely monitored for signs or symptoms of adverse reactions (see Interactions with Other Drugs section). .
Caution is advised when coadministering domperidone with drugs known to cause QT prolongation, and patients should be closely monitored for signs or symptoms of cardiovascular adverse reactions. Examples of such medicines:
- class IA antiarrhythmics (eg, disopyramide, quinidine);
- Class III antiarrhythmics (eg, amiodarone, dofetilide, dronedarone, ibutilide, sotalol);
- certain antipsychotics (eg, haloperidol, pimozide, sertindole);
- certain antidepressants (eg, citalopram, escitalopram);
- certain antibiotics (eg, levofloxacin, moxifloxacin);
- certain antifungals (eg, pentamidine);
- certain antimalarials (eg, halofantrine);
- certain gastrointestinal medicines (eg, dolasetron);
- certain anticancer drugs (eg, toremifene, vandetanib);
- some other medicines (eg, bepridil, methadone).
If the medicine has become unusable or has expired, do not throw it into wastewater or onto the street! Place the medication in a bag and place it in the trash. These measures will help protect the environment!
Impact on the ability to drive vehicles and machinery
Care must be taken when driving vehicles and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions due to the risk of developing adverse reactions that may affect these abilities.
How can I replace Motilium?
The most famous analogues and generics of the drug:
- Domrid;
- Domidon;
- Domperidone Hexal;
- Motinorm;
- Motoricum;
- Motilak;
- Peridonium.
You should not change the medicine to an alternative one on your own. In addition to differences in dosage, some of them have a wider list of contraindications; not all are approved for use in pediatrics.