Compound
The film-coated tablet contains 150/300 mg ranitidine hydrochloride .
Excipients: silicon dioxide (colloid), MCC (type 12), copovidone, Mg stearate. Film shell components (white Opadry AMB OY-B28920): soy lecithin, talc, xanthan gum, titanium dioxide, polyvinyl alcohol .
Solution for injection (1 ml) contains 0.025 grams of ranitidine hydrochloride .
pharmachologic effect
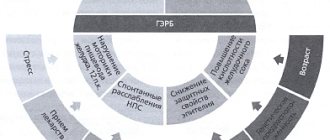
Ranitidine Akos is an antiulcer drug, the active substance of which belongs to the group of histamine H2 receptor antagonists. The principle of action is based on blocking H2 receptors in parietal cells located in the gastric mucosa, as well as inhibiting the production of hydrochloric acid . Under the influence of the active substance, the volume of total secretion decreases, suppressing the activity of pepsin in gastric juice .
Thanks to the antisecretory effect of Ranitidine, it is possible to create favorable conditions for the healing of ulcerative lesions in the digestive tract (stomach, duodenum). The active substance is capable of exerting a protective effect by enhancing reparative processes, increasing the secretion of special mucous substances, and improving microcirculation.
Pharmacological properties of the drug Ranitidine
Ranitidine is an antisecretory agent, an H2-histamine receptor antagonist. The mechanism of action is due to competitive reversible inhibition of the action of histamine on H2 receptors of the membranes of parietal cells of the gastric mucosa. Suppresses basal and stimulated secretion of hydrochloric acid, caused, in particular, by baroreceptor irritation (gastric distension), food load, the action of hormones and biogenic stimulants (gastrin, histamine, acetylcholine, pentagastrin, caffeine). Reduces pepsin activity. Strengthens the protective mechanisms of the gastric mucosa and promotes the healing of its damage caused by acid exposure, by reducing gastric secretion and increasing the formation of gastric mucus, the content of glycoproteins in it, stimulating the secretion of bicarbonate ions by the gastric mucosa, endogenous synthesis of prostaglandins in it and regeneration processes. The duration of action for a single oral dose is 12 hours. When taken orally, the bioavailability of ranitidine is approximately 50%. The maximum concentration in blood plasma is achieved 2–3 hours after oral administration. With intramuscular administration, the maximum concentration in blood plasma is achieved in the first 15 minutes after administration. Ranitidine is excreted primarily in the urine. Partially metabolized in the liver. The half-life is 2–3 hours. About 93% of the dose administered intravenously and 60–70% of the dose taken orally are excreted in the urine, the rest in feces. Approximately 70% of ranitidine administered intravenously and about 35% taken orally are excreted unchanged in the urine. Penetrates through the placenta and into breast milk.
Pharmacodynamics and pharmacokinetics
The active substance ranitidine is absorbed quite quickly from the lumen of the digestive tract. Food has no effect on the degree of absorption. Bioavailability reaches 50%. Peak concentrations are recorded within 2-3 hours after oral administration. 15% bound to plasma proteins. Partial metabolism takes place in the hepatic system with the formation of ranitidine S-oxide and desmethylranitidine .
The drug is characterized by a “first pass” effect through the hepatic system. The condition of the liver affects the extent and rate of elimination. After oral administration, the half-life is 2.5 hours, and with a creatinine clearance of 20-30 ml/min, this figure increases to 8-9 hours.
A small amount is excreted in the feces, the main part is excreted unchanged through the renal system. The active component does not pass the blood-brain barrier well, but penetrates the placenta well. Ranitidine is released during lactation.
Indications for use of Ranitidine
Ranitidine tablets - what do they help with? The main area of application of the drug is gastroenterology .
Ranitidine Akos - what does it help with? The drug is prescribed for the treatment of various pathologies of the digestive system, and can also be used for prophylactic purposes.
Indications for use of Ranitidine Acri
- symptomatic ulcerative lesions of the digestive tract;
- peptic ulcer of the digestive system (stomach, duodenum);
- Zollinger-Ellison syndrome;
- prevention of aspiration of gastric juice during surgical interventions with the introduction of anesthesia;
- prevention of the development of “stress” ulcers;
- reflux esophagitis;
- erosive esophagitis;
- prevention of the development of ulcerative lesions of the gastrointestinal tract after surgical interventions;
- prevention of recurrent bleeding from the upper digestive tract.
Indications for use of Ranitidine Sopharma are similar.
Ranitidine
Ranitidine
(lat.
ranitidine
) - an antiulcer drug, a blocker of histamine H2 receptors. Historically, it is the second (after cimetidine) antisecretory drug that suppresses acid production in the stomach. It is currently considered relatively old, has more side effects than modern drugs and is less effective than famotidine and proton pump inhibitors. In the fall of 2021, a number of drugs containing ranitidine were banned for use or approval for their use in clinical practice was temporarily suspended by regulators in many countries due to the presence or suspicion of the presence (the latter motivated by unsatisfactory testing of the drug for this ingredient) of potential carcinogen - N-nitrosodimethylamine.
The FDA, in its release dated April 1, 2021, required that all drugs containing ranitidine be removed from use and sale in US pharmacies due to the fact that when stored at temperatures above room temperature, drugs containing ranitidine may increase the amount of N-nitrosodimethylamine in some of them .
Ranitidine is a chemical compound
N-[2-[[5-(Dimethylaminomethyl)furfuryl]thio]ethyl]-N'-methyl-2-nitro-1,1-ethenediamine hydrochloride. Empirical formula: C13H22N4O3S.
Ranitidine is a drug
Ranitidine is the international nonproprietary name (INN) of the drug. According to the pharmacological index, it belongs to the group “II generation H2-histamine receptor blockers”. According to ATC, it belongs to the group “H2-histamine receptor blockers” and has the code A02BA02.
Pharmacodynamics of ranitidine
Ranitidine is a blocker of histamine H2 receptors on parietal cells of the gastric mucosa. Reduces basal and stimulated secretion of hydrochloric acid caused by irritation of baroreceptors, food load, the action of hormones and biogenic stimulants (gastrin, histamine, pentagastrin). Ranitidine reduces the volume of gastric juice and the content of hydrochloric acid in it, reduces the acidity of the stomach, which leads to a decrease in pepsin activity. The duration of action of ranitidine after a single dose is up to 12 hours.
Pharmacokinetics of ranitidine
When taken orally, the bioavailability of ranitidine is 50%. Plasma protein binding does not exceed 15%. Partially metabolized in the liver. Maximum concentrations of ranitidine in plasma are achieved 2 hours after taking film-coated tablets, 1 hour after taking effervescent tablets and range from 36 to 94 ng/ml. The half-life is 2-3 hours. About 30% of the administered dose of ranitidine is excreted unchanged in the urine, and a small amount is excreted in the feces. Penetrates through the placenta. Excreted in breast milk.
Professional medical work covering the treatment of gastrointestinal diseases with ranitidine
- Gorbakov V.V., Makarov Yu.S., Golochalova T.V. Comparative characteristics of antisecretory drugs of various groups according to daily pH monitoring // Attending physician. 2001. – No. 5–6.
- Yakovenko E.P. Zantac in the treatment of acid-related diseases. RGMU, Federal Gastroenterological Center, Moscow.
- Makhakova G.Ch., Dicheva D.T., Odintsova T.A. and others. Comparative characteristics of acid-suppressing drugs by conducting pharmacological tests with intragastric daily pH-metry // Attending physician. – 1999. – No. 6. – P. 24–26.
On the website gastroscan.ru in the literature catalog there is a section “H2-blockers”, containing articles devoted to the treatment of diseases of the gastrointestinal tract using H2-blockers of histamine receptors, including the use of ranitidine.
Indications for use of ranitidine
- treatment and prevention of exacerbations of gastric and duodenal ulcers;
- stomach and duodenal ulcers associated with taking NSAIDs;
- reflux esophagitis, erosive esophagitis;
- Zollinger-Ellison syndrome;
- treatment and prevention of postoperative, “stress” stomach ulcers;
- prevention of recurrent bleeding from the upper gastrointestinal tract;
- prevention of aspiration of gastric juice during operations under anesthesia (Mendelssohn syndrome).
Method of administration of ranitidine and dose
- Peptic ulcer of the stomach and duodenum. To treat exacerbations, ranitidine is prescribed 150 mg 2 times a day (morning and evening) or 300 mg at night. If necessary, 300 mg 2 times a day. The duration of treatment is 4-8 weeks. To prevent exacerbations, 150 mg is prescribed at night.
- Ulcers associated with NSAIDs. Prescribe 150 mg of ranitidine 2 times a day or 300 mg at night for 8-12 weeks. Prevention of ulcer formation when taking NSAIDs - 150 mg 2 times a day.
- Postoperative ulcers. Prescribe 150 mg of ranitidine 2 times a day for 4-8 weeks.
- Gastroesophageal reflux disease. Prescribe 150 mg 2 times a day or 300 mg at night. If necessary, the dose can be increased to 150 mg 4 times a day. The course of treatment is 8-12 weeks.
- Zollinger-Ellison syndrome. The initial dose is 150 mg ranitidine 3 times a day, the dose can be increased if necessary.
- Prevention of recurrent bleeding. 150 mg 2 times a day.
- Prevention of the development of Mendelssohn's syndrome. Ranitidine is prescribed at a dose of 150 mg 2 hours before anesthesia, and also, preferably 150 mg the night before.
Ranitidine is taken regardless of meals, without chewing, with a small amount of liquid.
Effervescent tablets are dissolved in a glass of water and drunk after complete dissolution.
For patients with renal failure with creatinine clearance less than 50 ml/min, the recommended dose is 150 mg of ranitidine per day.
Experience with the use of ranitidine in the treatment of functional heartburn
The updated American Gastroenterological Association functional heartburn guidelines (2020) present results from treating patients with functional heartburn with ranitidine 150 mg daily. It is noted that ranitidine has an analgesic effect by reducing the sensitivity of esophageal chemoreceptors to acid perfusion (Fass R., Zerbib F., Gyawali CP).
Precautions when using ranitidine
- It is undesirable to abruptly stop taking ranitidine due to the risk of relapse of peptic ulcer disease.
- Ranitidine should be used with caution in patients with impaired renal and hepatic function.
- Before starting treatment with ranitidine, it is necessary to exclude the possibility of a malignant disease of the esophagus, stomach or duodenum.
Contraindications to the use of ranitidine
Hypersensitivity to ranitidine or other components of the drug. Pregnancy, lactation. Children's age up to 14 years.
Side effects of ranitidine
- From the nervous system and sensory organs: headache, feeling of fatigue, dizziness, drowsiness, insomnia, vertigo, anxiety, depression; rarely - confusion, hallucinations (especially in elderly and weakened patients), reversible blurred vision, impaired accommodation of the eye.
- From the cardiovascular system and blood (hematopoiesis, hemostasis): arrhythmia, tachycardia, bradycardia, AV block, decreased blood pressure; reversible leukopenia, thrombocytopenia, granulocytopenia; rarely - agranulocytosis, pancytopenia, sometimes with bone marrow hypoplasia, aplastic anemia; sometimes - immune hemolytic anemia.
- From the gastrointestinal tract: nausea, vomiting, constipation, diarrhea, abdominal discomfort, pain; rarely - pancreatitis. Sometimes - hepatocellular, cholestatic or mixed hepatitis with/without jaundice (in such cases, ranitidine should be stopped immediately). These effects are usually reversible, but in rare cases they can be fatal. Rare cases of liver failure have also been reported. In healthy volunteers, AST concentrations were increased by at least 2-fold relative to pre-treatment levels in 6 of 12 people receiving 100 mg 4 times IV for 7 days and in 4 of 24 people receiving 50 mg 4 times IV for 5 days.
- From the musculoskeletal system: rarely - arthralgia, myalgia.
- Allergic reactions: skin rash, bronchospasm, fever, eosinophilia; rarely - erythema multiforme, anaphylactic shock, angioedema.
Ranitidine interactions
Antacids, sucralfate in high doses (2 g) slow down the absorption of ranitidine (when used simultaneously, the interval between taking antacids and ranitidine should be at least 1-2 hours).
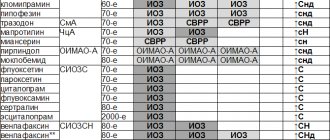
Smoking reduces the effectiveness of ranitidine. Additional prolongation of PT has been reported when ranitidine was coadministered with warfarin; however, in human pharmacokinetic studies at a ranitidine dose of 400 mg/day, no interaction was observed; ranitidine had no effect on the clearance of warfarin and PT; the possibility of interaction with warfarin at doses above 400 mg per day has not been studied. When administered twice daily with ranitidine and triazolam, plasma concentrations of triazolam were higher than when administered with triazolam alone. Triazolam AUC values in people 18–60 years of age were 10 and 28% higher after administration of ranitidine 75 and 150 mg tablets than after administration of triazolam alone. In patients over 60 years of age, AUC values were approximately 30% higher after taking ranitidine 75 and 150 mg tablets. Ranitidine increases the AUC (by 80%) and concentration (by 50%) of metoprolol in the blood serum, while the half-life of metoprolol increases from 4.4 to 6.5 hours. Reduces the absorption of itraconazole and ketoconazole (ranitidine should be taken 2 hours after taking them). Inhibits the metabolism of phenazone, hexobarbital, glipizide, buformin, BCC. Compatible with 0.9% sodium chloride solution, 5% dextrose solution, 0.18% sodium chloride solution and 4% dextrose solution, 4.2% sodium bicarbonate solution. When taken simultaneously with drugs that depress bone marrow, the risk of developing neutropenia increases. Possible interaction with alcohol. When taken together with loperamide, serious heart problems are possible (FDA Notice dated 6.7.2916).
Ranitidine overdose
- Symptoms: convulsions, bradycardia, ventricular arrhythmias.
- Treatment: induction of vomiting or gastric lavage, symptomatic therapy. For convulsions - intravenous diazepam, for bradycardia - atropine, for ventricular arrhythmias - lidocaine.
Use of ranitidine during pregnancy and lactation
Ranitidine is contraindicated for use during lactation. FDA category of risk for the fetus when used throughout pregnancy is B (animal studies have not revealed a risk of adverse effects on the fetus; there have been no adequate studies in pregnant women).
general information
By Decree of the Government of the Russian Federation of December 30, 2009 No. 2135-r, ranitidine (solution for intravenous and intramuscular administration; solution for injection; film-coated tablets; film-coated tablets) is included in the List of vital and essential medicines.
Trade names of drugs containing the active ingredient ranitidine
Having or having been registered in Russia: Asitek, Acidex, Atsilok, Vero-Ranitidine, Gistak, Zantac, Zantin, Zoran, Raniberl 150, Ranigast, Ranisan, Ranison, Ranitidine, Ranitidine-LekT, Ranitidine Vramed, Ranitidine SEDICO, Ranitidine-AKOS, Ranitidine-Acri, Ranitidine-BMS, Ranitidine-ratiopharm, Ranitidine-Ferein, Ranitidine hydrochloride, Ranitidine film-coated tablets, Ranitin, Rantag, Rantak, Ranks, Ulkodin, Ulran, Yazitin from the following manufacturers: Akrikhin HFC, Russia;
Bryntsalov-A, Russia; Wave International, India; Vector, Russia; Vector-Farm, Russia; Vertex, Russia; J.R. Sharma Overseas, India; Health UKR, Ukraine; Irbit Chemical and Pharmaceutical Plant, Russia; Kanonpharma production, Russia; Krka, Slovenia; Moskhimfarm-preparations named after. ON THE. Semashko, Russia; Natur Product, France; Natur Product Europe B.V., the Netherlands; Ozon, Russia; Olainfarm, Latvia; Panacea Biotech, India; Northern Star, Russia; Serena Pharma/Shreya Life Sciences, India; HEMOPHARM CONCERN A.D., Yugoslavia. The Zantac brand of ranitidine is registered in the USA: prescription Zantac 150 USP, Zantac 300 USP, Zantac 25 EFFERdose, Zantac Syrup USP and over-the-counter Zantac 75, which has a reduced content of ranitidine - 75 mg and is approved for self-treatment of heartburn.
Instructions from manufacturers
Some instructions for the medical use of drugs with the only active ingredient - ranitidine (pdf):
- For Russia:
- "Ranitidine-Akri", film-coated tablets, 150 mg, JSC "Akrikhin",
- "Zantac 150 (ranitidine hydrochloride) Tablets, USP. Zantac 300 (ranitidine hydrochloride) Tablets, USP. Zantac 25 (ranitidine hydrochloride effervescent) EFFERdose Tablets, USP. Zantac (ranitidine hydrochloride) Syrup, USP. Prescribing Information". GlaxoSmithKline, January 2011
Ranitidine has contraindications, side effects and application features; consultation with a specialist is necessary. Back to section
Side effects
Hematopoietic system:
- leukopenia (with long-term therapy);
- thrombocytopenia.
The cardiovascular system:
- development of atrioventricular block (rarely, mainly with intravenous infusion).
Digestive tract:
- stool disorders ( constipation / diarrhea syndrome );
- hepatitis (extremely rare).
Side effects from the central nervous system:
- vertigo , dizziness ;
- fast fatiguability;
- blurred visual perception;
- headache;
- hallucinations (extremely rare);
- confusion (extremely rare).
Endocrine system, metabolism:
- increased prolactin ;
- increased creatinine ;
- amenorrhea;
- gynecomastia;
- decreased libido ;
- impotence.
Other reactions:
- recurrent mumps ;
- arterial hypotension;
- bronchospasm;
- arthralgia;
- hair loss;
- anaphylactic shock;
- angioedema;
- hives;
- various rashes on the skin;
- myalgia.
Ranitidine tablets, instructions for use (Method and dosage)
The treatment regimen is selected individually. The tablets are intended to be taken orally.
Daily dosage is 300-450 mg (can be increased to 600-900 mg if necessary), divided into 2-3 doses. To prevent exacerbation of diseases of the digestive tract, the drug is prescribed at bedtime at a dose of 150 mg. The duration of therapy is determined by the dynamics of the disease.
For pathology of the renal system, the medication is prescribed twice a day at a dose of 75 mg. Instructions for use of Ranitidine Akos are similar. Your doctor will tell you how long you can take the pills (on average, the course of treatment is 2-4 weeks).
How to take ranitidine?
Ranitidine is available in the form of tablets, effervescent tablets and granules, and syrup for oral administration. It is usually taken once a day before bedtime or 2-4 times a day. To prevent the symptoms that cause heartburn, it is taken 30-60 minutes before eating.
Ranitidine should be taken exactly as prescribed by your doctor. You cannot independently change the dosage or frequency of administration per day.
Dosing ranitidine in the form of syrup is necessary only using the measuring cup that comes with the package.
Ranitidine effervescent tablets and granules should be dissolved in one glass of water, approximately 180-240 ml.
Overdose
Main manifestations:
- skin rashes;
- confusion;
- headache;
- dizziness;
- increased drowsiness.
First aid consists of taking enterosorbents ( Polysorb , Smecta , Activated Carbon and others), calling an ambulance.
What are the side effects of ranitidine?
Ranitidine may exhibit the following side effects :
- headache, fatigue, dizziness;
- nausea, dry mouth, vomiting;
- diarrhea or constipation;
- on laboratory blood tests - a decrease in blood cells (platelets, leukocytes, erythrocytes);
- decreased blood pressure, slow pulse;
- joint and/or muscle pain;
- blurred vision.
Ranitidine may cause other side effects. You may need help from a healthcare professional if you experience any unusual problems while taking this medication.
Interaction
There is a decrease in ranitidine absorption rates when treated with antacids . Elderly patients experience deterioration in attention and memory while taking anticholinergic drugs . It is assumed that medications that block histamine H2 receptors are able to suppress the ulcerogenic effect of drugs from the NSAID on the gastric mucosa. There is a decrease in the clearance of Warfarin during treatment with Ranitidine. Medical practice describes a case of bleeding and hypoprothrombinemia in a patient who was taking Warfarin .
There may be an undesirable increase in the absorption rates of ranitidine during simultaneous therapy with bismuth tripotassium dicitrate . Cases of hypoglycemia when taking Glibenclamide .
Ranitidine inhibits the absorption of Itraconazole and Ketoconazole . The half-life of Metoprolol and its AUC increase during treatment with Ranitidine. The absorption of the drug changes when taking high doses of Sucralfate (more than 2 g).
There is a slowdown in the excretion of Procainamide through the renal system, which leads to an increase in the concentration of the active substance in the blood. The absorption of Triazolam increases, which is associated with a change in the pH of gastric juice. The risk of toxicity increases with treatment with Phenytoin , which is explained by a significant increase in its concentration in the blood. There is an increase in the bioavailability of Furosemide with simultaneous therapy with Ranitidine.
In the medical literature there is a description of a case of the development of ventricular arrhythmia of the bigeminy in a patient who took Ranitidine and Quinidine . When treated with Cisapride, of cardiotoxicity increases . There is an increase in the level of Cyclosporine in the blood with parallel treatment with Ranitidine.
Symptoms and consequences of ranitidine overdose
If you take too high a dose of ranitidine, an overdose of this drug may occur, which includes the following unpleasant symptoms and negative consequences:
- involuntary movements (convulsions);
- decreased heart rate (bradycardia);
- a small number of red blood cells in the blood, due to hemolytic anemia;
- Stagnation of bile in the liver - cholestatic hepatitis.
If you observe such conditions, you need the help of a doctor to carry out symptomatic treatment.
special instructions
In case of severe pathology of the renal system, the medication is prescribed with caution. Before using the drug, it is necessary to exclude oncological diseases of the intestines , esophagus and stomach .
Long-term therapy of weakened patients who are in a state of stress can provoke the development of bacterial disease of the stomach, as well as the subsequent spread of the inflammatory process.
If the medication is abruptly discontinued, the risk of relapse of peptic ulcer increases. Preventive therapy is more effective with a course of taking the drug for 45 days in the fall and spring, compared to continuous use.
In patients suffering from various rhythm disorders, rapid intravenous administration of the solution can provoke bradycardia . For persons with of porphyria , Ranitidine is prescribed with caution due to the risk of developing an acute attack.
Distortion of laboratory test parameters (liver enzymes, creatinine, GGT) is allowed. The time interval between taking antacids and Ranitidine should be at least 1-2 hours due to the risk of changes in the absorption of the active substance. Clinical studies confirming the safety of the drug in pediatric practice are limited.
What should you not do while taking ranitidine?
During the period of drug therapy with ranitidine, it is recommended to avoid drinking alcoholic beverages, as well as foods that increase stomach acidity.
It is advisable to stop smoking, as tobacco reduces the effectiveness of ranitidine, which may require increasing the dose of the medication.
Ranitidine should not be stopped abruptly, as “rebound syndrome” may occur, in which case it will cause an increase in acidity and, as a result, an exacerbation of the disease.
Ranitidine analogs
Level 4 ATC code matches:
Gastrosidine
Gistak
Acylok
Cimetidine
Zantac
Kvamatel
Famotidine
Structural analogues:
- Zantac;
- Gistak;
- Acylok;
- Ranisan.
Reviews of Ranitidine
The drug allows you to quickly relieve pain in the epigastric region due to ulcerative pathology of the digestive tract, gastropathy by reducing the acidity of gastric juice. Reviews about Ranitidine are mostly positive, because... The drug is well tolerated, causing virtually no negative symptoms if the dosage regimen is followed. Among the advantages are the low cost of the tablets and the quick effect in relieving heartburn.
The medication can be used in emergency cases in case of errors in the diet to prevent exacerbation of gastritis and peptic ulcers.
The downside is that it is impossible to use the medication during pregnancy and lactation.
What you need to know before you start taking ranitidine
If you are allergic to ranitidine, you should stop using it and consult your doctor to find an alternative treatment for conditions associated with stomach acid.
It is recommended that you avoid drinking alcohol during treatment with ranitidine. This may increase the risk of stomach damage. It may take up to 8 weeks of taking this medicine for the ulcer to heal. For best results, you should continue to use ranitidine as directed by your doctor. Consultation with your doctor is necessary if your symptoms do not improve after 6 weeks of treatment.
If, when treating heartburn with ranitidine, it does not go away for more than 2 weeks, it is advisable to consult a doctor.
You should be careful and consult your doctor about taking ranitidine if you have a history of the following conditions:
- porphyria (hereditary disease caused by impaired heme synthesis with an increase in the content of porphyrin intermediates in the blood and tissues);
- phenylketonuria (congenital disorder of amino acid metabolism);
- liver and/or kidney diseases.
Be careful! Heartburn can mimic the early symptoms of a heart attack. If you experience heartburn, you should pay attention to the presence of additional symptoms, such as chest pain or a feeling of heaviness, pain that spreads to the arm or shoulder, nausea, sweating and general malaise.
Ranitidine should not be taken during pregnancy or while breastfeeding. If you have a peptic ulcer, you need to consult a doctor to find the safest treatment for you.
Ranitidine is not suitable for use by anyone under 12 years of age.
Note! Using ranitidine may increase the risk of developing pneumonia. Symptoms of pneumonia include chest pain, fever, feeling short of breath, and a cough with green or yellow sputum.
Ranitidine price, where to buy
The cost of tablets varies depending on the region of sale and pharmacy chain. The average price of Ranitidine in Russia is 30 rubles.
- Online pharmacies in RussiaRussia
- Online pharmacies in UkraineUkraine
- Online pharmacies in KazakhstanKazakhstan
LuxPharma* special offer
- Ranitidine TEVA (Zantac) tablet 300 mg 30 pcs
RUB 1,580 order - Zantac ampoules (Ranitidine in ampoules, Zantac) injection solution 25 mg/ml 2 ml No. 5
1970 rub. order
show more
Pharmacy24
- Ranitidine 150 mg N30 tablets PrAT "Tekhnolog", Uman, Cherkasy region, Ukraine
18 UAH. order - Ranitidine 150 mg N20 tablets PrAT "Technolog", Uman, Cherkasy region, Ukraine
12 UAH order
- Ranitidine-Darnitsa 0.15g No. 10 tablets PrAT” Pharmaceutical company “Darnitsa”, Ukraine
8 UAH order
- Ranitidine-Darnitsa 0.15g N20 tablets PrAT” Pharmaceutical company “Darnitsa”, Ukraine
15 UAH order
PaniPharmacy
- Ranitidine tablets Ranitidine tablets 150 mg No. 20 Ukraine, Tekhnolog ChAO
13 UAH order
- Ranitidine tablets Ranitidine-Zdorovye Forte tablets. p/o 300 mg No. 20 Ukraine, Health LLC
28 UAH order
- Ranitidine tablets Ranitidine-Zdorovye Forte tablets. p/o 300 mg No. 10 Ukraine, Health LLC
15 UAH order
- Ranitidine tablets Ranitidine tablets. p/o 0.15g No. 20 Ukraine, Darnitsa ChAO
19 UAH order
- Ranitidine tablets Ranitidine tablets. p/o 0.15g No. 10 Ukraine, Health LLC
8 UAH order
show more