Esomeprazole
The drug is used orally, parenterally intravenously.
The drug in the form of film-coated tablets is used orally. The tablet should be swallowed whole with liquid. Tablets should not be chewed or broken. For patients with difficulty swallowing, you can dissolve the tablets in half a glass of still water.
Adults and children from 12 years old
Gastroesophageal reflux disease
Treatment of erosive reflux esophagitis: 40 mg once a day for 4 weeks. An additional 4-week course of treatment is recommended in cases where, after the first course, healing of esophagitis does not occur or symptoms persist.
Long-term maintenance treatment after healing of erosive reflux esophagitis to prevent relapse: 20 mg once a day.
Symptomatic treatment of gastroesophageal reflux disease: 20 mg once daily in patients without esophagitis. If symptoms do not disappear after 4 weeks of treatment, the patient should be further examined. After eliminating the symptoms, you can switch to the “as needed” regimen of the drug, i.e. take 20 mg once daily when symptoms return. For patients taking NSAIDs who are at risk of developing gastric or duodenal ulcers, treatment on an 'as needed' basis is not recommended.
Adults
Peptic ulcer of the stomach and duodenum
As part of combination therapy for Helicobacter pylori eradication:
treatment of duodenal ulcer associated with Helicobacter pylori: the drug 20 mg, amoxicillin 1 g and clarithromycin 500 mg. All drugs are taken twice a day for 1 week.
Prevention of relapse of peptic ulcers associated with Helicobacter pylori: drug 20 mg, amoxicillin 1 g and clarithromycin 500 mg. All drugs are taken twice a day for 1 week.
Long-term acid suppression therapy in patients who have suffered bleeding from a peptic ulcer (after intravenous use of drugs that reduce the secretion of gastric glands to prevent relapse) 40 mg of the drug 1 time per day for 4 weeks after the end of intravenous therapy with drugs that reduce the secretion of gastric glands.
Patients taking NSAIDs for a long time:
- healing of stomach ulcers associated with taking NSAIDs: 20 mg or 40 mg once a day. The duration of treatment is 4-8 weeks.
- prevention of gastric and duodenal ulcers associated with taking NSAIDs: 20 mg or 40 mg once a day.
Conditions associated with pathological hypersecretion of the gastric glands, including Zollinger-Ellison syndrome and idiopathic hypersecretion: The recommended starting dose is 40 mg twice daily. In the future, the dose is selected individually, the duration of treatment is determined by the clinical picture of the disease.
If it is impossible to carry out oral therapy, patients may be recommended to prescribe the drug parenterally at a dose of 20-40 mg once a day, for which a lyophilisate is used to prepare a solution for intravenous administration.
Esomeprazole, 40 mg, enteric-coated tablets, 14 pcs.
Esomeprazole is the S-isomer of omeprazole and reduces gastric acid secretion by specifically inhibiting the proton pump in the parietal cells of the gastric mucosa. The S- and R-isomers of omeprazole have similar pharmacodynamic activities.
Mechanism of action
Esomeprazole is a weak base that transforms into an active form in the highly acidic environment of the secretory tubules of the parietal cells of the gastric mucosa where it inhibits the proton pump - the enzyme H + / K + -ATPase, thereby inhibiting both basal and stimulated secretion of hydrochloric acid.
Effect on the secretion of hydrochloric acid in the stomach.
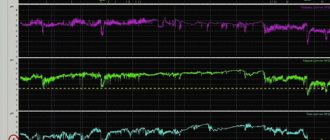
After taking esomeprazole orally at a dose of 20 mg or 40 mg, the therapeutic effect develops within 1 hour. When taking the drug daily for 5 days at a dose of 20 mg once a day, the average maximum acid production after stimulation with pentagastrin is reduced by 90% (when measured 6-7 hours after taking the drug on the 5th day of therapy).
In patients with gastroesophageal reflux disease (GERD) and the presence of clinical symptoms, after 5 days of daily oral esomeprazole 20 mg or 40 mg, intragastric pH values above 4 were maintained for an average of 13 and 17 hours out of 24 hours. While taking esomeprazole at a dose of 20 mg per day, an intragastric pH value above 4 was maintained for at least 8, 12 and 16 hours in 76%, 54% and 24% of patients, respectively. For 40 mg esomeprazole, this ratio is 97%, 92% and 56%, respectively.
A correlation was identified between the concentration of the drug in plasma and the inhibition of hydrochloric acid secretion (to assess the concentration, the AUC parameter was used - the area under the concentration-time curve).
The therapeutic effect achieved by inhibiting the secretion of hydrochloric acid.
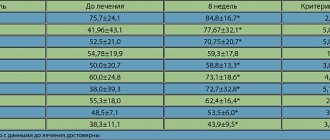
When taking esomeprazole at a dose of 40 mg, healing of reflux esophagitis occurs in approximately 78% of patients after 4 weeks of therapy and in 93% after 8 weeks of therapy.
Treatment with esomeprazole 20 mg twice daily in combination with appropriate antibiotics for one week results in successful eradication of Helicobacter pylori in approximately 90% of patients.
Patients with uncomplicated duodenal ulcer after a weekly course of eradication therapy do not require subsequent monotherapy with drugs that reduce the secretion of gastric glands to heal the ulcer and eliminate symptoms.
The effectiveness of esomeprazole for peptic ulcer bleeding was demonstrated in a study in patients with endoscopically confirmed peptic ulcer bleeding.
Other effects associated with inhibition of hydrochloric acid secretion
During treatment with drugs that reduce the secretion of gastric glands, the concentration of gastrin in the plasma increases as a result of a decrease in acid secretion. Due to decreased secretion of hydrochloric acid, the concentration of chromogranin A (CgA) increases. Increased concentrations of CgA may affect the results of examinations for the detection of neuroendocrine tumors. To prevent this effect, therapy with proton pump inhibitors should be suspended 5-14 days before testing CgA concentrations. If during this time the CgA concentration has not returned to normal, the study should be repeated.
In children and adult patients receiving esomeprazole for a long time, an increase in the number of enterochromaffin-like cells is observed, probably associated with an increase in the concentration of gastrin in plasma. This phenomenon has no clinical significance.
Patients taking drugs that reduce the secretion of gastric glands for a long period of time are more likely to experience the formation of glandular cysts in the stomach. These phenomena are caused by physiological changes as a result of pronounced inhibition of hydrochloric acid secretion. Cysts are benign and undergo reverse development.
The use of drugs that suppress the secretion of hydrochloric acid in the stomach, including proton pump inhibitors, is accompanied by an increase in the content of microbial flora in the stomach that is normally present in the gastrointestinal tract. The use of proton pump inhibitors may lead to a slight increase in the risk of infectious diseases of the gastrointestinal tract caused by bacteria of the genus Salmonella spp. and Campylobacter spp. and in hospitalized patients, Clostridium difficile is likely.
In two comparative studies with ranitidine as the active comparator, esomeprazole was shown to be more effective in healing gastric ulcers in patients receiving nonsteroidal anti-inflammatory drugs (NSAIDs), including selective cyclooxygenase-2 (COX-2) inhibitors.
In two studies, esomeprazole showed high efficacy in preventing gastric and duodenal ulcers in patients receiving NSAIDs (age group over 60 years and/or with a history of peptic ulcers) including selective COX-2 inhibitors.
Nexium
Esomeprazole is the S-isomer of omeprazole and reduces gastric acid secretion by specifically inhibiting the proton pump in gastric parietal cells. The S- and R-isomers of omeprazole have similar pharmacodynamic activities.
Mechanism of action
Esomeprazole is a weak base that transforms into an active form in the highly acidic environment of the secretory tubules of the parietal cells of the gastric mucosa and inhibits the proton pump - the enzyme H + / K + - ATPase, thereby inhibiting both basal and stimulated secretion of hydrochloric acid.
Effect on the secretion of hydrochloric acid in the stomach
The effect of esomeprazole develops within 1 hour after oral administration of 20 mg or 40 mg. When taking the drug daily for 5 days at a dose of 20 mg once a day, the average maximum concentration of hydrochloric acid after stimulation with pentagastrin is reduced by 90% (when measuring the acid concentration 6-7 hours after taking the drug on the 5th day of therapy). In patients with gastroesophageal reflux disease (GERD) and the presence of clinical symptoms, after 5 days of daily oral esomeprazole 20 mg or 40 mg, intragastric pH values above 4 were maintained for an average of 13 and 17 hours out of 24 hours. When taking esomeprazole at a dose of 20 mg per day, an intragastric pH value above 4 was maintained for at least 8, 12 and 16 hours in 76%, 54% and 24% of patients, respectively. For 40 mg esomeprazole, this ratio is 97%, 92% and 56%, respectively.
A correlation was found between the concentration of the drug in plasma and the inhibition of hydrochloric acid secretion (the AUC parameter (area under the concentration-time curve) was used to assess the concentration).
The therapeutic effect achieved by inhibiting the secretion of hydrochloric acid. When taking Nexium at a dose of 40 mg, healing of reflux esophagitis occurs in approximately 78% of patients after 4 weeks of therapy and in 93% after 8 weeks of therapy.
Treatment with Nexium at a dose of 20 mg 2 times a day in combination with appropriate antibiotics for one week leads to successful eradication of Helicobacter pylori in approximately 90% of patients.
Patients with uncomplicated peptic ulcer disease after a week-long eradication course do not require subsequent monotherapy with drugs that reduce the secretion of gastric glands to heal the ulcer and eliminate symptoms.
The effectiveness of Nexium for peptic ulcer bleeding was demonstrated in a study of patients with endoscopically confirmed peptic ulcer bleeding.
Other effects associated with inhibition of hydrochloric acid secretion. During treatment with drugs that reduce the secretion of gastric glands, the concentration of gastrin in the plasma increases as a result of a decrease in acid secretion. Due to a decrease in the secretion of hydrochloric acid, the concentration of chromogranin A (CgA) increases. Increased concentrations of CgA may affect the results of examinations for the detection of neuroendocrine tumors. To prevent this effect, therapy with proton pump inhibitors should be suspended 5-14 days before testing CgA concentrations. If during this time the CgA concentration has not returned to normal, the study should be repeated.
In children and adult patients receiving esomeprazole for a long time, there is an increase in the number of enterochromaffin-like cells, probably associated with an increase in plasma gastrin concentrations. This phenomenon has no clinical significance.
Patients taking drugs that reduce the secretion of gastric glands for a long period of time are more likely to develop glandular cysts in the stomach. These phenomena are caused by physiological changes as a result of pronounced inhibition of hydrochloric acid secretion. Cysts are benign and undergo reverse development.
The use of drugs that suppress the secretion of hydrochloric acid in the stomach, including proton pump inhibitors, is accompanied by an increase in the content of microbial flora in the stomach that is normally present in the gastrointestinal tract. The use of proton pump inhibitors may lead to a slight increase in the risk of infectious diseases of the gastrointestinal tract caused by bacteria of the genus Salmonella spp. and Campylobacter spp. and, in hospitalized patients, probably Clostridium difficile.
In two comparative studies with ranitidine, Nexium was shown to be superior in healing gastric ulcers in patients treated with nonsteroidal anti-inflammatory drugs (NSAIDs), including selective cyclooxygenase-2 (COX-2) inhibitors. In two studies, Nexium showed high efficacy in the prevention of gastric and duodenal ulcers in patients receiving NSAIDs (age group over 60 years and/or with a history of peptic ulcers), including selective COX-2 inhibitors.
Pharmacokinetics
Absorption and distribution
Esomeprazole is unstable in an acidic environment, so tablets containing granules of the drug, the shell of which is resistant to the action of gastric juice, are used for oral administration. Under in vivo conditions, only a small portion of esomeprazole is converted to the R-isomer. The drug is rapidly absorbed: maximum plasma concentration is reached 1-2 hours after administration. The absolute bioavailability of esomeprazole after a single dose of 40 mg is 64% and increases to 89% with daily dosing once daily. For a dose of 20 mg esomeprazole, these figures are 50% and 68%, respectively. The volume of distribution at steady state concentration in healthy people is approximately 0.22 l/kg body weight. Esomeprazole is 97% bound to plasma proteins.
Eating slows down and reduces the absorption of esomeprazole in the stomach, but this does not have a significant effect on the effectiveness of inhibition of hydrochloric acid secretion.
Metabolism and excretion
Esomeprazole is metabolized via the cytochrome P450 system. The main part is metabolized with the participation of a specific polymorphic isoenzyme CYP2C19, resulting in the formation of hydroxylated and demethylated metabolites of esomeprazole. The remainder is metabolized by the CYP3A4 isoenzyme; this produces a sulfo derivative of esomeprazole, which is the main metabolite detected in plasma.
The parameters given below mainly reflect the nature of pharmacokinetics in patients with increased activity of the CYP2C19 isoenzyme. The total clearance is approximately 17 l/h after a single dose of the drug and 9 l/h after repeated doses. The half-life is 1.3 hours when taken systematically once a day. The area under the concentration-time curve (AUC) increases with repeated dosing of esomeprazole. The dose-dependent increase in AUC with repeated administration of esomeprazole is non-linear, which is a consequence of a decrease in first-pass metabolism through the liver, as well as a decrease in systemic clearance, probably caused by inhibition of the CYP2C19 isoenzyme by esomeprazole and/or its sulfo derivatives. When taken daily once a day, esomeprazole is completely eliminated from the blood plasma in the interval between doses and does not accumulate.
The main metabolites of esomeprazole do not affect the secretion of gastric acid. When administered orally, up to 80% of the dose is excreted in the form of metabolites in the urine, the rest is excreted in feces. Less than 1% unchanged esomeprazole is found in urine.
Features of pharmacokinetics in some groups of patients.
Approximately 2.9±1.5% of the population has reduced activity of the CYP2C19 isoenzyme. In these patients, esomeprazole is metabolized primarily through the action of CYP3A4. When systematically taking 40 mg of esomeprazole once a day, the average AUC value is 100% higher than the value of this parameter in patients with increased activity of the CYP2C19 isoenzyme. The average values of maximum plasma concentrations in patients with reduced isoenzyme activity are increased by approximately 60%. These features do not affect the dose and method of administration of esomeprazole. In elderly patients (71-80 years), the metabolism of esomeprazole does not undergo significant changes.
After a single dose of 40 mg esomeprazole, the average AUC value in women is 30% higher than that in men. When taking the drug daily once a day, there are no differences in pharmacokinetics between men and women. These features do not affect the dose and method of administration of esomeprazole. In patients with mild to moderate hepatic impairment, the metabolism of esomeprazole may be impaired. In patients with severe hepatic impairment, the metabolic rate is reduced, resulting in a 2-fold increase in the AUC value for esomeprazole.
Pharmacokinetic studies have not been conducted in patients with renal failure. Since it is not esomeprazole itself that is excreted through the kidneys, but its metabolites, it can be assumed that the metabolism of esomeprazole in patients with renal failure does not change.
In children aged 12-18 years, after repeated administration of 20 mg and 40 mg of esomeprazole, the AUC and TCmax values in the blood plasma were similar to the AUC and TCmax values in adults.