Pharmacodynamics and pharmacokinetics
Pharmacodynamics
norepinephrine and serotonin reuptake blocker , it also slightly inhibits dopamine ; does not have significant affinity for dopamine, histamine, cholinergic and adrenergic receptors The mechanism of action is to inhibit or stop the reuptake of norepinephrine and serotonin , resulting in increased noradrenergic and serotonergic neurotransmission in the brain.
It has a central mechanism for weakening pain, which is manifested by an increase in the pain threshold in neurogenic pain syndrome .
Pharmacokinetics
Well absorbed from the intestines, the highest concentration is achieved six hours after consumption. Taking with food indirectly reduces absorption by 11%.
Plasma protein binding reaches 90%. It is actively transformed with the participation of the enzymes CYP1A2 and CYP2D6 , resulting in the synthesis of two metabolites ( 5-hydroxy,6-methoxyduloxetine sulfate, 4-hydroxyduloxetine glucuronide ). The half-life reaches 12 hours. Excreted in urine.
Contraindications
- Sensitization to the components of the drug.
- Simultaneous use with MAO inhibitors.
- Decompensated angle-closure glaucoma.
- Age less than 18 years.
- Fructose intolerance , sucrase deficiency, glucose-galactose malabsorption.
- Severe liver diseases.
- Concomitant use of strong CYP1A2 enzyme inhibitors.
- Severe form of chronic renal failure .
- Arterial hypertension.
- Age less than 18 years.
It is recommended to use Cymbalta with caution for seizures, bipolar disorder and mania, intraocular hypertension , increased risk of hyponatremia , suicidal thoughts, liver and kidney dysfunction.
special instructions
When serotonin reuptake inhibitors were prescribed in combination with MAO inhibitors, cases of serious reactions, sometimes fatal, were observed (hyperthermia, rigidity, myoclonus, various disorders with possible sharp fluctuations in vital signs and changes in mental status, including severe agitation leading to delirium and to whom). Such reactions are also possible in cases where the serotonin reuptake inhibitor was discontinued shortly before the administration of MAO inhibitors (symptoms may develop, including those characteristic of neuroleptic malignant syndrome).
The effects of combined use of duloxetine and MAO inhibitors have not been evaluated in either humans or animals. The use of Cymbalta simultaneously with MAO inhibitors or within 14 days after their discontinuation is not recommended, because Duloxetine is a serotonin and norepinephrine (norepinephrine) reuptake inhibitor. MAO inhibitors should not be administered for at least 5 days after discontinuation of duloxetine.
As with other drugs that have similar effects on the central nervous system, duloxetine should be used with caution in patients with a history of manic episodes, as well as a history of epileptic seizures.
Depressive conditions are accompanied by a high risk of developing suicidal behavior. As a result, patients diagnosed with depression who are taking duloxetine should inform their physician of any disturbing thoughts and feelings.
While using the drug, mydriasis may develop; caution should be exercised when prescribing duloxetine to patients with increased intraocular pressure or in persons at risk of developing acute angle-closure glaucoma.
In patients with severe renal impairment (creatinine clearance <30 ml/min) or severe liver failure, an increase in plasma duloxetine concentrations is observed. If duloxetine is clinically justified in such patients, the drug should be used at lower initial doses.
In patients with arterial hypertension and/or other diseases of the cardiovascular system, it is recommended to monitor blood pressure.
While using the drug, patients should be careful when drinking alcohol.
Impact on the ability to drive vehicles and operate machinery
Patients taking duloxetine should be careful when engaging in potentially hazardous activities (due to the possible occurrence of drowsiness).
Side effects
- More often than others, patients using duloxetine experience headache, nausea, drowsiness, dry mouth, and dizziness.
- laryngitis may occur .
- Changes in the immune system: hypersensitivity , anaphylactic reaction.
- Changes in the hormonal sphere: hypothyroidism .
- Metabolic changes: dehydration, decreased appetite, hyperglycemia, hyponatremia, paradoxical release syndrome of antidiuretic hormone .
- Mental changes: anxiety, insomnia , agitation, decreased libido , unusual dreams, impaired orgasm, sleep disorders, suicidal thoughts, bruxism , disorientation, apathy, mania , aggression, hallucinations, hostility.
- Changes in nervous activity: headache, dyskinesia , drowsiness, dizziness, tremor, dysgeusia, paresthesia, myoclonus, akathisia, lethargy , excitability, impaired concentration, serotonin syndrome , psychomotor agitation, decreased sleep quality, convulsions , extrapyramidal disorders.
- Changes in sensory organs: mydriasis, vertigo , blurred vision, glaucoma , tinnitus, dry eyes, ear pain.
- Changes in the circulation: supraventricular arrhythmia, tachycardia, hyperemia, hypertension, orthostatic hypotension, cold extremities, fainting.
- Changes in breathing: pain in the oropharynx, yawning , nosebleeds.
- Digestive changes: nausea, diarrhea , dry mouth, vomiting, constipation, dyspepsia, abdominal pain gastroenteritis, gastritis, belching, dysphagia , bad breath, stomatitis, hematochezia, hepatitis, liver damage, jaundice .
- Skin changes: rash, increased sweating, itching , urticaria , night sweats, contact dermatitis, photosensitivity , cold sweat, Stevens-Johnson syndrome, Quincke's edema.
- Changes in the musculoskeletal system: muscle stiffness, musculoskeletal pain, muscle spasms, cramps, trismus.
- Changes in the genitourinary system: urinary retention, increased frequency of urination, icturia , polyuria, dysuria, impaired ejaculation, erectile dysfunction, changes in the menstrual cycle, gynecological bleeding, testicular pain, galactorrhea, menopausal symptoms, hyperprolactinemia.
- General and local disorders: increased fatigue, impaired taste, atypical sensations, chest pain, feeling of cold, chills, thirst, malaise, gait disturbance.
- Laboratory and physical findings: weight change; increased levels of ALT, alkaline phosphatase, AST, bilirubin, GGT, CPK; increased levels of potassium and cholesterol in the blood .
Abrupt withdrawal of the drug often causes withdrawal syndrome , which is manifested by: dizziness, headache, sensory disturbances, tremor, diarrhea, sleep disturbance, weakness, drowsiness, irritability, anxiety, nausea.
Instructions for use of Cymbalta (Method and dosage)
Instructions for use of Cymbalta require swallowing the capsules whole without chewing. Do not add the product to food or mix it with liquids.
The recommended initial dose is 60 mg once daily. In some patients, to achieve a better result, it is necessary to increase the dose to 120 mg per day divided into 2 doses.
In patients with renal failure in the terminal stage, the initial dose is 30 mg of the drug once a day.
In patients with liver damage, the initial dosage of the drug should be reduced or the frequency of administration in patients with cirrhosis .
Do not suddenly discontinue drug therapy. When stopping treatment, the dosage should be slowly reduced over 10-14 days to reduce the risk of withdrawal symptoms .
Cymbalta (duloxetine) is a representative of the newest generation of antidepressants. This drug can be classified as the fifth generation of antidepressants. Antidepressants “come from” tuberculosis, because in the 1940s. The euphoric effect of isonicotinic acid hydroxide was noted in severe tuberculosis patients with bleeding. It was later shown that this effect is due to the irreversible blockade of monoamine oxidase (MAO), an enzyme that destroys biogenic amines (including serotonin) in the synaptic cleft. The next step was the discovery of tricyclic antidepressants (TCAs), which can only be considered accidental to a certain extent. Subsequently, new generations of antidepressants were created thanks to knowledge of the stages of life of the mediator [2]:
- synthesis;
- secretion;
- accumulation;
- release into the synaptic cleft;
- reception;
- destruction in the synaptic cleft;
- reverse capture.
It should be taken into account that biogenic amines - dopamine, norepinephrine, serotonin - have much in common both in the above-described stages of the life of mediators (including common enzyme systems of destruction), and from the point of view of their importance in the pathogenesis of many neurological and psychiatric diseases.
TCAs have been shown to be serotonin, dopamine, and norepinephrine reuptake inhibitors. They have proven to be much more effective and safer in the treatment of depression than irreversible MAO blockers, and for many decades they were the “gold standard” for antidepressants. The advent of TCAs led to MAO blockers leaving clinical practice and returning only with the discovery of reversible MAO blockers such as moclobemide. Moreover, these drugs began to be considered as selective inhibitors of MAO type A, mainly involved in the destruction of serotonin.
The TCA ideology was further developed in selective serotonin reuptake inhibitors (SSRIs). These drugs have undoubted advantages, such as high efficiency, sufficient safety, and the absence of the need for dose titration and withdrawal syndrome. All this has made them very popular not only in psychiatric, but also in general therapeutic practice. SSRIs have also been shown to be highly effective in the treatment of anxiety disorders and this has led to their use in the treatment of generalized anxiety and panic disorders.
Another group of drugs are antidepressants that affect reception by blocking postsynaptic or presynaptic receptors. These include, in particular, mianserin, an antagonist
a2-adrenergic receptors and serotonin receptors (5HT1A and 5HT2C), and trazodone is a 5HT2 receptor blocker.
Thus, until the mid-1990s. The main trend in the development of antidepressants was their selectivity towards monoamine mediators. However, it was subsequently shown that additional therapeutic possibilities lie in the combination of inhibition of serotonin and norepinephrine reuptake, which allows accelerating the onset of the antidepressant effect and affecting pain [8]. All this led to the creation of dual-action antidepressants that selectively block the reuptake of serotonin and norepinephrine. The first representative of this class was venlafaxine. Cymbalta (duloxetine) is a new representative of the class of dual-acting antidepressants.
According to the mechanism of action, Cymbalta is a balanced and powerful inhibitor of the reuptake of serotonin and norepinephrine [12]. The drug is one of the antidepressants with the strongest affinity (affinity) for the transport subunits of serotonin and norepinephrine. Balance means approximately equal binding of transport subunits that perform the reuptake of these mediators.
The affinity coefficient K is estimated in vitro as the drug concentration required to inhibit 50% of the maximum binding sites determined by the radioligand method. The lower the K coefficient, the smaller the amount of drug needed to inhibit binding sites and the stronger the affinity of the drug for transport subunits. According to this indicator, Cymbalta is 3 times superior to venlafaxine [12].
Restoring the balance between serotonergic and noradrenergic mediation with Cymbalta plays a critical role in the treatment of depression and pain, given the role of imbalance of these mediators in the formation of emotional and pain disorders.
Cymbalta has virtually no effect on dopamine reuptake blockade and does not interact with cholinergic and histaminergic receptors.
The pharmacokinetics of Cymbalta is linear and dose dependent. The maximum concentration in plasma is reached after 6 hours, and after food intake - after 10 hours [11]. The half-life of the drug in plasma is 12 hours. Cymbalta is metabolized in the liver through isoenzymes 2D6 (primary metabolic pathway) and 1A2 of the cytochrome P450 system. Cymbalta is classified as a moderate inhibitor of the 2D6 isoenzyme. More than 90% of the drug is protein bound, and this should be taken into account when co-prescribing Cymbalta with substances that may compete with it for this binding. Cymbalta should be combined with caution with TCAs (2D6 inhibition) and antiarrhythmic drugs. Cymbalta does not inhibit isoenzymes 1A2 and 3A4, which mediate the metabolism of most drugs. Therefore, in neurological practice, the use of Cymbalta practically does not limit the use of other drugs. Cymbalta metabolites are inactive and 70% are excreted in the urine. Age and gender have little effect on the pharmacokinetics of the drug, which is why dosage adjustment is not required depending on these indicators.
The clinical effectiveness of Cymbalta against depression can be considered fully studied, since placebo-controlled and comparative studies have been conducted on the use of this drug in adult patients diagnosed with major depressive disorder (in accordance with DSM-IV criteria) [9]. Already in the first week of treatment with Cymbalta at a dose of 60 mg, a significant improvement in the well-being of patients on the Clinical Global Impression (CGI) scale was observed in comparison with the placebo group [3, 4]. The level of depression on the Hamilton scale under the influence of Cymbalta began to significantly decrease from the second week of treatment. At the end of therapy, the effect of Cymbalta was 1.5 times greater than the effect of placebo.
Undoubtedly, the most important indicator for any drug is its safety. As part of the studies, more than 11,500 patients received Cymbalta. The initial dose of Cymbalta is usually equal to the therapeutic dose and is 60 mg/day (single dose) [4, 5]. To reduce the severity of side effects in the first week of use, you can take the drug at a dose of 30 mg/day. Cymbalta is prescribed to patients over 18 years of age. Currently available data indicate that there are no differences in the effectiveness or tolerability of Cymbalta in older and younger patients. It is not recommended to use the drug in patients with severe renal and hepatic pathology [11].
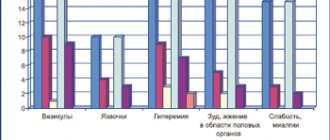
In general, Cymbalta is well tolerated, and the spectrum of its side effects is close to the safety profile of other modern antidepressants. In clinical studies, the following side effects were most often observed [3, 10]:
- nausea (20%);
- dry mouth (15%);
- constipation (11%);
- decreased appetite (8%);
- increased fatigue (8%);
- drowsiness (7%);
- increased sweating (6%).
As a rule, nausea was the reason for discontinuation of the drug, which occurred in 1% of cases. Using Cymbalta 30 mg during the first week reduces the likelihood of developing this side effect.
Taking into account the noradrenergic activity of Cymbalta, the most serious attention in its study was paid to the activity of the cardiovascular system. The drug was shown to not cause negative cardiovascular effects: heart rate increased by two beats per minute compared to placebo, and there was no connection between the increase in blood pressure and the dose of Cymbalta. However, it is recommended to monitor blood pressure at the beginning of treatment. There were no significant changes in the ECG.
Undoubtedly, depression plays a very important role in neurology and occurs in a wide variety of diseases. Depression accompanying somatic or neurological diseases further worsens the quality of life of patients and is more difficult to treat [1, 6]. Moreover, depression can significantly modify the course of the underlying disease, and the use of antidepressants seems to “clear” its clinical picture.
In stroke patients, depression increases the risk of disability and mortality [1]. Patients with chronic neurological diseases are generally more susceptible to depression than patients with somatic pathology. Neurological diseases in which depression can occur are very numerous [1, 2]:
- cerebrovascular diseases;
- chronic pain syndromes of various origins;
- extrapyramidal diseases - Parkinson's disease, Huntington's chorea, etc.;
- Alzheimer's disease and other dementias;
- multiple sclerosis;
- brain tumors;
- epilepsy;
- consequences of traumatic brain injury;
- encephalopathy of endocrine origin (with hypothyroidism, thyrotoxicosis).
The list goes on, and there is an undeniable pattern: the longer and more severe the neurological disease, the greater the degree of disability of the patient, the higher the risk of depression and its severity.
It should also be emphasized that a number of drugs have prodepressant activity:
- neuroleptics;
- barbiturates;
- sleeping pills and sedatives;
- chemotherapeutic agents;
- interferons;
- H2 blockers;
- non-steroidal anti-inflammatory drugs;
- muscle relaxants;
- sulfonamides;
- corticosteroids;
- drugs that change sex hormone levels;
- antihypertensive drugs;
- calcium antagonists;
- benzodiazepines.
Based on the foregoing, it is quite obvious that Cymbalta, as a drug with a proven rapid-onset effect and the possibility of combination with other drugs used in neurological practice, will become widespread. It is important to emphasize that Cymbalta has a pronounced therapeutic effect against pain syndromes associated with depression, which has been shown in a large number of clinical studies. Many of them tested the dynamics of pain intensity using a visual analogue scale (VAS) and a somatic symptom questionnaire (Somatic Symptom Inventory - SSI). Cymbalta demonstrated high efficacy for pain on both scales (VAS and SSI). For example, the intensity of back pain under the influence of Cymbalta significantly decreased already in the first week of treatment and significantly regressed in the 2-3rd week of therapy [3]. Upon completion of treatment, the severity of almost all pain syndromes decreased, with the exception of headache [6]. The analgesic effect was independent of the reduction in emotional disorders and was 50% a direct effect of Cymbalta.
Cymbalta has been surprisingly effective for neuropathic pain. The mechanism of this phenomenon is not completely clear, but it can be assumed that the analgesic effect of the drug is associated with an increase in the activity of antinociceptive (anti-pain) systems, as a result of which the perception of pain is weakened. To date, two studies have been completed to study the analgesic effectiveness of Cymbalta in diabetic neuropathy [6]. The persistence of pain in diabetes mellitus is explained by the development of “central sensitization” due to the trigger activity of affected peripheral sensory neurons. Central sensitization involves changes in the nociceptive pathways of the central nervous system, resulting in hyperalgesia (increased sensitivity to painful stimuli), allodynia (the experience of non-painful stimuli as painful), and the sensation of pain when unstimulated.
In these studies, Cymbalta was used for 12 weeks at a dose of 60 mg/day. Compared with placebo, while taking Cymbalta, pain significantly regressed, and the improvement was observed already in the first week of treatment and persisted throughout the entire period of taking the drug. At the same time, no significant differences were identified between Cymbalta and placebo in terms of glucose metabolism.
Thus, Cymbalta is a new highly effective and fairly safe antidepressant, which will undoubtedly occupy an important place in neurological practice, having not only antidepressant, but also analgesic activity, especially in situations of intractable neuropathic pain.
Overdose
Symptoms of overdose: coma , drowsiness , clonic convulsions , vomiting, serotonin syndrome, tachycardia.
Treatment of overdose: gastric lavage, taking enterosorbents ; if serotonin syndrome corrective therapy with Cyproheptadine . It is recommended to monitor cardiac activity along with the prescription of symptomatic treatment.
Interaction
Due to the risk of serotonin syndrome, the drug should not be used together with MAO and for another two weeks after stopping taking MAO inhibitors.
Co-administration with potential CYP1A2 and CYP1A2 enzymes may cause an increase in drug levels.
Caution should be used when used together with other drugs that affect the nervous system, including alcohol.
In rare cases, when used simultaneously with other serotonin uptake inhibitors and serotonergic drugs, may occur .
Caution should be exercised when using Cymbalta with drugs metabolized by the CYP2D6 enzyme system.
Concomitant use with anticoagulants may provoke bleeding associated with pharmacodynamic interactions.
Use of the drug during pregnancy and lactation
The use of the drug during pregnancy is possible only in cases where the expected benefit to the mother outweighs the potential risk to the fetus, because There is insufficient clinical experience with the use of duloxetine during pregnancy.
If it is necessary to use the drug during lactation, the issue of stopping breastfeeding should be decided (due to lack of experience in use).
Patients should be warned that if they become pregnant or plan to become pregnant while using duloxetine, they should inform their doctor.
Analogs
Level 4 ATC code matches:
Pipofezin
Bethol
Incazan
Melitor
Azafen
Miaser
Velafax
Mirtazonal
Venlaxor
Remeron
Venlafaxine
Lerivon
Mirtazapine
Velaxin
Coaxil
Pyrazidol
Deprim
Gelarium Hypericum
Negrustin
Trittico
Duloxetine Canon, Alventa, Velaxin, Venlift, Gelarium Hypericum, Deprim Forte, Coaxil, Melitor, Mirazep, Neuroplant, Trittico.
Cymbalta price, where to buy
Buying Cymbalta 30 mg No. 14 in Russia will cost 880-970 rubles.
In Ukraine, the price of this form of release starts from 750 hryvnia.
- Online pharmacies in RussiaRussia
ZdravCity
- Cymbalta capsules 30 mg 14 pcs. Lilly del Caribe Inc.
/Lilly S.A 1810 rub. order - Cymbalta capsules 60 mg 28 pcs. Lilly del Caribe Inc. /Lilly S.A
RUR 3,623 order
- Cymbalta capsules 60 mg 14 pcs. Lilly del Caribe Inc. /Lilly S.A
1806 RUR order
Release form, composition and packaging
capsules
, size No. 1, opaque, blue/green, with a dosage of “60 mg” printed in white ink and identification code “9542”; the contents of the capsules are pellets from white to grayish-white.
| 1 caps. | |
| duloxetine (as hydrochloride) | 60 mg |
Excipients:
sucrose, hypromellose, granulated sugar (no more than 91.5% sucrose, starch), talc, hypromellose acetate succinate, triethyl citrate, white dye (titanium dioxide, hypromellose).
Shell composition:
indigo carmine, titanium dioxide, sodium lauryl sulfate, gelatin, iron oxide yellow dye.
7 pcs. - blisters (1) - cardboard packs. 7 pcs. - blisters (2) - cardboard packs. 7 pcs. - blisters (4) - cardboard packs. 14 pcs. - blisters (1) - cardboard packs. 14 pcs. - blisters (2) - cardboard packs. 14 pcs. - blisters (6) - cardboard packs.