Directions for use and doses
Externally. Apply exactly to the treated area with a special applicator or a small thin wooden stick, avoiding contact with adjacent areas and mucous membranes.
Small papillomas (up to 2 mm) and filiform warts: lubricate once.
Larger papillomas and small warts (2–3 mm): apply 3–4 times, taking breaks to allow the solution to dry.
Warts on the hands and soles: treat 7–10 times at intervals of 3–4 minutes.
Keratomas and dry calluses: treat 3-4 times with an interval of 3-4 minutes.
Before removing warts on the hands with a dense keratinized surface, plantar warts, keratomas and dry calluses, they are softened with keratolytic ointment (for example, salicylic), covering the lubricated area with compress paper or plastic film and, on top of them, with a gauze bandage. Possible sealing with adhesive tape. Then the bandage or plaster is removed, the skin is steamed in hot water with the addition of soap and soda for 10–15 minutes, and the horny layers are cut off with nail scissors or tongs. The drug is applied to dried skin several times at intervals of 3–4 minutes for drying.
If it is necessary to apply the drug repeatedly, in order to avoid burns to the surrounding skin, it is advisable to lubricate it with zinc paste. The paste is removed with a dry gauze swab after the last portion of the drug has dried.
Genital warts: removed in the treatment room (not recommended on your own), applying the drug to each element separately 1-2 times with an interval of 3-4 minutes.
Repeated treatment, if necessary, is carried out 6–8 days after the crust falls off. The procedure can be repeated 4–5 times.
Verrukacid 2g solution for external use
pharmachologic effect
It has a cauterizing effect and coagulates skin proteins.
Composition and release form Verrukacid 2g solution for external use
Solution - 1 g:
- Active ingredients: phenol 588 mg, metacresol 392 mg.
- Excipients: ethanol 95% 10 mg, purified water up to 1 g.
2 g - dark glass bottles (1) complete with or without applicators - cardboard packs.
Description of the dosage form
Solution for external use; in the form of a mobile oily liquid from pinkish or light brown to brown with the smell of phenol.
Directions for use and doses
Verrucacid is for external use only.
The drug is applied precisely to the treated area with a special applicator or a small thin wooden stick, preventing it from coming into contact with adjacent healthy areas of skin and mucous membranes.
For small papillomas (up to 2 mm in size) and filiform warts, Verrucacid is applied once.
Larger papillomas and small warts (2-3 mm in size) are smeared with the drug 3-4 times, taking breaks to allow the applied liquid to dry.
Before removing warts with a dense keratinized surface on the hands, plantar warts, keratomas, and dry calluses with Verrucacid, it is necessary to remove horny layers from their surface. To do this, apply a keratolytic ointment (for example, 10% salicylic or other) for several hours, covering the lubricated area with compress paper or plastic film, and then use a gauze bandage. Possible sealing with adhesive tape. After this, the bandage or adhesive plaster is removed, the skin is steamed in hot water with the addition of soap and soda for 10-15 minutes and the horny layers are removed (cut off with nail scissors or tongs). Verrucacid is applied to dried skin several times, taking 3-4 minute breaks to allow the drug to dry.
Warts on the hands and soles are treated with Verrucacid 7-10 times with an interval of 3-4 minutes.
It is enough to treat keratomas and dry calluses 3-4 times with an interval of 3-4 minutes.
If it is necessary to apply the drug repeatedly, in order to avoid burns to the surrounding skin, it is advisable to lubricate it with zinc paste. The paste is removed with a dry gauze swab after the last portion of Verrukacid has dried.
It is not recommended to remove genital warts yourself; they are treated with Verrucacid in the treatment room of the clinic by a dermatovenereologist or urologist. The drug Verrukacid is applied to each element separately 1-2 times with an interval of 3-4 minutes.
Repeated treatment, if necessary, is carried out 6-8 days after the crust falls off. 4-5 procedures are allowed.
Indications for use Verrukacid 2g solution for external use
- Common, filiform and plantar warts;
- papillomas;
- genital warts of the skin;
- dry calluses;
- keratomas.
Contraindications
- Increased individual sensitivity to the components of the drug;
- pigmented nevi (moles);
- rashes located on the red border of the lips and mucous membranes;
- Do not apply the drug to the surface of the skin with an area of more than 20 cm2;
- children's age up to 7 years.
Application of Verrukacid 2g solution for external use during pregnancy and breastfeeding
The use of the drug is possible in cases where the expected benefit to the mother outweighs the potential risk to the fetus or child. During lactation, it is not recommended to remove formations located on the mammary glands and hands.
Use in children
Contraindicated for children under 7 years of age.
special instructions
Do not allow the drug to come into contact with mucous membranes, especially the mucous membrane of the eyes. In case of contact, immediately rinse eyes with plenty of water and consult an ophthalmologist.
It is unacceptable to bandage the areas treated with the drug, seal them with a plaster, lubricate them with any ointments and remove the crust; Do not reapply the drug before the dates indicated above. Clothing made of synthetic fabrics should not touch the area of skin treated with the drug.
It is not recommended to apply Verrucacid to formations located in skin folds (inguinal folds, anal area, interdigital spaces, etc.) and heavily sweating areas in order to avoid burns to unaffected skin of the contacting surface or as a result of the drug spreading over wet skin.
The area of skin treated with Verrucacid must be air-dried; it must not be lubricated with any ointments or washed with water on the first day after treatment.
When used correctly, the drug does not leave scars.
Particular care and caution is required when treating children with the drug.
Overdose
Not identified.
Side effects Verrukacid 2g solution for external use
Burn (in case of contact with healthy skin). If the drug accidentally comes into contact with healthy skin, it is necessary to immediately, carefully, without rubbing, remove it from the skin, and then treat the affected areas with 10-40% ethyl alcohol or alcohol-containing liquids (vodka, lotion, cologne) and wash thoroughly with warm water and soap. If a burn occurs, it is recommended to use anti-burn and healing agents. Swelling and redness of the skin when applied to the eye area, which goes away on its own. Allergic reactions.
Drug interactions
The components of the drug easily dissolve in the ointment base, and therefore it is not recommended to lubricate the skin area treated with Verrucacid with any ointments.
Precautionary measures
Avoid contact with healthy skin and mucous membranes (especially eyes). In case of contact with eyes, rinse them immediately with plenty of water and consult an ophthalmologist.
Do not bandage, tape or lubricate the treated areas with ointment, remove crusts, or reapply the drug before the specified time. It is necessary to ensure that the treated area dries in the air, to prevent its contact with clothing made of synthetic fabric, and not to wash it on the first day.
It is not recommended to apply Verrucacid to formations located in skin folds (inguinal folds, anal area, interdigital spaces, etc.) and heavily sweating areas, in order to avoid burns to unaffected skin.
If it is necessary to apply the drug repeatedly, in order to avoid burns to the surrounding skin, it is advisable to lubricate it with zinc paste. The paste is removed with a dry gauze swab after the last portion of Verrukacid has dried.
If the drug accidentally comes into contact with healthy skin, it is necessary to immediately, carefully, without rubbing, remove it from the skin, and then treat the affected areas with 10–40% ethyl alcohol or alcohol-containing liquids (lotion, cologne) and wash thoroughly with warm water and soap.
If a burn occurs, it is recommended to use anti-burn and healing agents.
Particular care should be taken when using the drug in children.
Registration number:
P N001835/01.
Trade name:
Verrukacid®.
INN or group name:
phenol, metacresol.
Dosage form:
solution for external use.
Compound:
- Active ingredients:
phenol – 588 mg, 3-methylphenol (metacresol) – 392 mg. - Excipients:
ethyl alcohol (ethanol) 95% - 10 mg, purified water up to 1 g.
Description:
mobile oily liquid from pinkish or light yellow to brown with the smell of phenol.
Pharmacotherapeutic group:
local necrotizing agent.
ATX code
(D11AF).
Pharmacological properties
It has a cauterizing effect and coagulates skin proteins.
Indications for use
Common, filiform and plantar warts, papillomas, genital warts of the skin, dry calluses, keratomas.
Contraindications
Increased individual sensitivity to the components of the drug. Pigmented nevi (moles), rashes located on the red border of the lips and mucous membranes. Do not apply the drug to the skin surface area of more than 20 cm2. Children under 7 years old.
Pregnancy and lactation period
The use of the drug is possible in cases where the expected benefit to the mother outweighs the potential risk to the fetus or child. During lactation, it is not recommended to remove formations located on the mammary glands and hands.
Directions for use and dosage
Verrukacid® is intended for external use only.
The drug is applied accurately
onto the treated area with a special applicator or a small thin wooden stick, avoiding it coming into contact with adjacent healthy areas of skin and mucous membranes. For small papillomas (up to 2 mm in size) and filamentous warts, Verrukacid® is applied once. Larger papillomas and small warts (2-3 mm in size) are smeared with the drug 3-4 times, taking breaks to allow the applied liquid to dry.
Before removing warts with a dense keratinized surface on the hands, plantar warts, keratomas, and dry calluses with Verrucacid®, it is necessary to remove horny layers from their surface. To do this, apply a keratolytic ointment (for example, 10% salicylic or other) for several hours, covering the lubricated area with compress paper or plastic film, and then use a gauze bandage. Possible sealing with adhesive tape. After this, the bandage or adhesive plaster is removed, the skin is steamed in hot water with the addition of soap and soda for 10-15 minutes and the horny layers are removed (cut off with nail scissors or tongs). Verrukacid® is applied to dried skin several times, taking 3-4 minute breaks to allow the drug to dry.
Warts on the hands and soles are treated with Verrukacid® 7-10 times with an interval of 3-4 minutes.
It is enough to treat keratomas and dry calluses 3-4 times with an interval of 3-4 minutes.
If it is necessary to apply the drug repeatedly, in order to avoid burns to the surrounding skin, it is advisable to lubricate it with zinc paste. The paste is removed with a dry gauze swab after the last portion of Verrukacid® has dried.
It is not recommended to remove genital warts yourself; they are treated with Verrucacid® in the treatment room of the clinic by a dermatovenereologist or urologist. The drug Verrukacid® is applied to each element separately 1-2 times with an interval of 3-4 minutes.
Repeated treatment, if necessary, is carried out 6-8 days after the crust falls off. 4-5 procedures are allowed.
Side effects
Burn (in case of contact with healthy skin). If the drug accidentally comes into contact with healthy skin, it is necessary to immediately, carefully, without rubbing, remove it from the skin, and then treat the affected areas with 10-40% ethyl alcohol or alcohol-containing liquids (vodka, lotion, cologne) and wash thoroughly with warm water and soap. If a burn occurs, it is recommended to use anti-burn and healing agents.
Swelling and redness of the skin when applied to the eye area, which goes away on its own.
Allergic reactions.
Overdose
Not identified.
Interaction with other drugs
The components of the drug easily dissolve in the ointment base, and therefore it is not recommended to lubricate the skin area treated with Verrucacid® with any ointments.
special instructions
Do not allow the drug to come into contact with mucous membranes, especially the mucous membrane of the eyes. In case of contact, immediately rinse eyes with plenty of water and consult an ophthalmologist.
It is unacceptable to bandage the areas treated with the drug, seal them with a plaster, lubricate them with any ointments and remove the crust; Do not reapply the drug before the dates indicated above. Clothing made of synthetic fabrics should not touch the area of skin treated with the drug.
It is not recommended to apply Verrucacid® to formations located in skin folds (inguinal folds, anal area, interdigital spaces, etc.) and heavily sweating areas in order to avoid burns to unaffected skin of the contacting surface or as a result of the drug spreading over wet skin.
The area of skin treated with Verrucacid® must be air-dried; it must not be lubricated with any ointments or washed with water on the first day after treatment.
When used correctly, the drug does not leave scars.
Particular care and caution is required when treating children with the drug.
Release form
Solution for external use in dark glass bottles with and without applicator, 2 and 10 g.
Each bottle, along with instructions for medical use, is placed in a cardboard pack.
Additionally, each cardboard pack can include a plastic lid with an applicator.
Storage conditions
In a place protected from light at a temperature of 15 to 25 ° C.
Keep out of the reach of children.
Best before date
5 years. Do not use after expiration date.
Conditions for dispensing from pharmacies
Dispensed without a prescription.
Experience of using Vartox cream paste in monotherapy of viral warts
Iskra A.S., Iskra E.L. Medical, St. Petersburg, Russia Family Medicine Center named after I.P. Pavlova, St. Petersburg, Russia
Data are provided on the clinical types of warts associated with various types of human papillomavirus. The methods used to treat warts are analyzed: destructive, external therapy, combined. We present our own experience in the treatment of viral warts with a modern drug for external therapy, which is Vartox cream paste, Russia. The 40% urea (urea) included in the Vartox cream paste has a powerful keratolytic effect - it softens the surface of the wart for the penetration of the additional antiviral component of the 0.1% glycyrrhizic acid paste. Glycyrrhizic acid 0.1% has an antiviral effect, including against human papillomaviruses. By interacting with the virus, glycyrrhizic acid destroys the development phases of the virus and has an immunostimulating effect. The effectiveness of treatment of viral warts was 76% (38 out of 50 patients). Clinical cure was observed in both primary and recurrent nature of the process. The treatment was characterized by the absence of side effects and complications, low trauma, good tolerance, complete tissue restoration without cosmetic defects.
Key words: viral warts, Vartox cream paste, external therapy for the treatment of warts.
The human papillomavirus is ubiquitous. It causes a whole range of benign lesions of the skin and mucous membranes and plays an important role in the pathogenesis of malignant neoplasms. Asymptomatic carriage of the virus also occurs. More than 190 types of human papillomavirus have been described, which are classified into high and low oncogenic risk groups according to their potential to induce cancer. Among the skin diseases caused by the human papillomavirus, three are particularly common. These are simple warts, plantar warts and flat warts. Simple ones account for about 60–70% of all warts and affect almost 20% of children. Plantar warts account for about 20–30%; they are common in teenagers. Flat warts occur in people of any age, accounting for less than 5%. Some types of human papillomavirus selectively infect the epidermis. Among the skin diseases caused by the human papillomavirus, the most common are warts. The disease is a localized benign hyperplasia of the epidermis and manifests itself as papules or plaques [6–8].
Etiology . The human papillomavirus belongs to the Papovaviridae family; it is a DNA-containing virus that multiplies in the nuclei of the epithelium, which leads to the development of infectious acanthosis [8].
Infection . Occurs by contact (when skin comes into contact with affected skin); penetration of infection is facilitated by minor injuries that disrupt the integrity of the stratum corneum of the epidermis. Infection is possible through simple contact in areas of skin trauma, in the gym, or in the swimming pool.
Risk factors . Immunodeficiency, such as HIV infection and immunosuppressive therapy. Risk group: children, elderly people, pregnancy, complicated medical history. Without treatment, warts last for years.
Complaints . The main complaint is a cosmetic defect. Plantar warts cause pain, especially when walking. Minor injuries may cause bleeding.
Simple warts (common types of viruses 3 and 10). Synonyms: verruca vulgaris, common wart, common wart. Elements of the rash. Dense papules with a diameter of 1–10 mm (occasionally even larger); the surface is covered with cracks, horny layers, and vegetations. When localized on the fingers and palms, the skin pattern becomes distorted and disappears. Restoration of fingerprints is a sign of recovery. The color does not differ from the surrounding skin. Dermatoscopy reveals black-brown dots, which are thrombosed dark vessels (capillaries). This is a pathognomonic sign of warts. The shape is round, polycyclic. Arrangement: single element or multiple isolated elements arranged randomly. Near the removed wart, new ones may appear, located in a circle. Localization. Favorite localization is easily injured areas, that is, hands, fingers. Plantar warts (HPV types 1, 2, 4). Synonyms: verruca plantaris, horny wart. Elements of the rash. First, a small shiny papule with clear boundaries, then a keratinizing plaque with a rough, uneven surface. Against its background, small black-brown dots are visible - thrombosed capillaries. The skin pattern is distorted, its restoration is a sign of recovery. Plantar warts heal without scarring. On the contrary, after cryodestruction and electrocoagulation, scars often remain for life. The color does not differ from the surrounding skin. To identify the characteristic black-brown dots, you need to remove the horny masses from the surface with a scalpel. Palpation. Warts located in areas of pressure are painful. Location: small elements merge to form a “mosaic” wart. “Kissing” warts are found on the touching surfaces of the fingers. Localization. Soles: in the projection of the heads of the metatarsal bones, heels, toe pads, and other supporting areas of the foot. Usually a single formation, but 2 or more warts occur. Flat warts. Synonyms: verruca plana, juvenile wart. Elements of the rash. Flat, clearly demarcated papules with a smooth surface, 1–6 mm in diameter. They rise above the surface of the skin by 1–2 mm. The color is light brown, pink or normal skin color. Shape: round, oval, polygonal. In place of scratching - linear. Location: always multiple isolated elements arranged in a cluster. In places of injury, a linear arrangement is possible.
Localization. Favorite localization is the face, especially the chin; dorsum of hands; shins [1–6].
Course and prognosis.
In patients with good immunity, viral warts may resolve on their own without the use of additional medications. In case of immunodeficiency, treatment is necessary, and many methods are often ineffective [2, 3].
Today, there are many topical preparations for removing warts. Drug treatment: cauterizing chemicals, coagulating, local keratolytics, antiviral and immune external agents. In this article we will consider one of the options for drug treatment using the example of Vartox cream paste. External therapy with Vartox cream paste according to the scheme 1 time per day on the area of the formation under a cotton swab and a patch, daily until the formation is completely resolved. Cryodestruction. Carry out when drug treatment is ineffective. Use a stick with a cotton swab or cryodestructor “Frost” for 15–30 s. Liquid nitrogen is applied to the area of formation, covering up to 1–2 mm of healthy tissue. Cryodestruction is repeated several times at intervals of 2–4 weeks. until complete recovery. Several procedures are possible. Freezing destroys only the affected tissue. A significant disadvantage of the method is pain. It can be combined with external therapy before and after the procedure, including the use of Vartox cream paste. Electrocoagulation. More effective than cryodestruction, but more often accompanied by scarring. Before removing flat warts, the skin is lubricated with lidocaine cream; if warts are localized on the palms and soles, local anesthesia is needed. Laser therapy. Used when other methods are ineffective. A carbon dioxide laser or picosecond laser is used. Surgical treatment if the above methods are ineffective. Rationale for the feasibility of using Vartox cream paste: this drug contains urea at a concentration of 40%; urea, or carbamide, acts on the wart so that it gradually becomes soft and can be easily removed with a pumice stone or a scraper. Composition of the cream paste: in addition to 40% urea, or carbamide, Vartox cream paste also contains glycyrrhizic acid in a concentration of 0.1%. Urea allows you to speed up the process of softening a benign formation on the sole and prevents possible complications after its removal. Glycyrrhizic acid has an antiviral effect. Information about the mechanism of action of Vartox cream paste.
The topical preparation Vartox cream paste very effectively fights warts on the soles and palms.
How to use Vartox:
1. The cream paste is applied in a thick layer to the affected area of the foot or palm (hand).
2. Secure with cotton wool and adhesive tape.
3. Removed after a day, the softened wart is removed with pumice.
4. The area is washed with water without using soap.
5. The procedure can be repeated several times until clinical symptoms disappear.
Indications for use. Cream paste is actively used to treat warts of various origins.
Main indications for use of Vartox cream paste: ƒ
- benign formations (viral warts); ƒ
- calluses; ƒ
— preparation for cryodestruction or laser procedures; ƒ
- removal of keratinized skin after removing a wart in another way.
This all relates to the main indications, but, as mentioned above, the drug is used to remove other forms of formations on the skin. For example, warts on the hands may also be an indication for their removal with a urea-based cream paste. But before use, it is necessary to conduct an allergy test. To do this, apply a small amount of cream paste to the skin of the wrist and wait about 15 minutes. If no pathological changes have occurred, there is no itching, there are no rashes, you can safely use Vartox to remove benign growths on the hands. Vartox can be used in the presence of single or multiple plantar warts. In this case, it is necessary to follow a certain order of treatment if there are several formations on the sole at the same time.
Contraindications for use. Among the absolute contraindications for using Vartox cream paste are: ƒ
- malignant or unspecified formations on the skin; ƒ
— the presence of allergic reactions to individual components of the paste; ƒ
— traumatization of a wart, an open wound; ƒ
- trophic ulcers, bedsores.
Relative contraindications: ƒ
- sensitive skin; ƒ
— itching, redness after applying the cream paste; ƒ
- varicose veins on the legs.
Side effects: not pronounced, some of them occur in people with sensitive skin.
These include: ƒ
- itching, severe peeling of the skin around the site of application of the drug; ƒ
- redness, swelling, burning; ƒ
- slight pain during the process of resorption of the wart.
These side effects are not dangerous to health, but rather are a common consequence of using cream paste to remove any formations. If such reactions do not go away within a few hours, you should consult a dermatologist.
Description of diagnostic methods: ƒ
— clinical examination by a dermatologist; ƒ
— dermatoscopy; ƒ
- additional examination methods - general blood test;
— infectious group: antibodies to HIV-1 and 2 and HIV-1 and 2 antigen, syphilis RPR, Anti-HCV, HBsAg;
— quantitative determination of HPV DNA of 15 types.
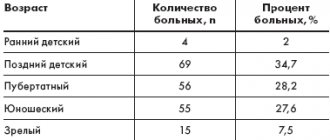
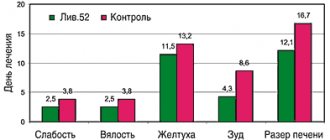
On the basis of the Medical and Family Medicine Center named after. I.P. Pavlov we observed 50 patients with plantar and palmar warts. All patients completed an anonymous survey. Of these, 20 patients had photographs of formations before and after treatment. All patients were asked to use only external therapy (monotherapy) in the form of Vartox cream paste for 2 weeks to 3 months, depending on the clinical picture and degree of damage, number and size of formations. 60% used external therapy for up to 1 month (in 20% of patients the treatment was 2 weeks, in 40% - 1 month), the remaining 40% used the drug for up to 3 months - of which: 10% - 6 weeks, 10% - 8 weeks, 12% – 10 weeks, 8% – 12 weeks. The study included both men and women aged 18 to 50 years with lesions of the palms and soles. We set ourselves the following tasks: 1. Adhere to monotherapy with Vartox cream paste. 2. Treatment of patients for no more than 3 months, but not less than 2 weeks. 3. Patients had to be examined at least twice and at least once after the entire course of treatment. 4. The study recruited patients of different ages and genders. 5. Patients underwent photographic recording before, during and after treatment with Vartox and a questionnaire. 6. The drug was used only on the skin of the palms and soles. As an indicator of the treatment outcome, we used the dynamics of pain syndrome, subjective sensations, visual (cosmetic) and palpation data of local manifestations of the disease throughout the entire period of use of Vartox cream-paste monotherapy. The intensity of subjective symptoms (burning sensation, pain) was assessed on the following scale: significant severity of symptoms – 6 points; average severity – 3 points; mild severity – 1 point, absence of symptoms – 0. The final assessment of all subjective symptoms was made using a composite index, which is the sum of the scores of all symptoms divided by the number of patients. The summary index was calculated at the 1st and 2nd visits. Clinical tolerability was assessed by patients in questionnaires as good, moderate and poor. No special statistical analysis methods were used. The initial total index of all patients was 142 points. After the 1st visit, the index decreased by 2 times and was 71 points, on the 2nd visit – 30 points. All patients assessed well the tolerability of the drug during the questionnaire. „
1. Vartox cream-paste is a safe remedy for the treatment of various types of warts. During our research, not a single side effect was noted on the skin or other organs and systems. The effectiveness was high and amounted to 76% (38 patients out of 50). In 12 patients (24%), no positive effect of treatment was observed, and hardware methods for removing formations were recommended.
2. The drug is effective when used in monotherapy for the treatment of plantar and palmar warts.
3. The components of this product are well tolerated by patients.
4. The drug can be used daily and for a long time until clinical symptoms resolve under the supervision of a dermatologist, as well as in combination with other methods of destruction or in preparation for them. 5. The drug is not a replacement for laser treatment or cryodestruction, but is an alternative drug treatment or an addition to hardware methods. 6. We recommend the drug as a conservative treatment in dermatological practice for the treatment of warts.
1. Gorlanov I., Zaslavskij D., Milyavskaya I., Leina L., Olovyannikov O., Kulikova S. (2017) Uchebnik “Detskaya dermatoloverologiya”. GEOTAR-MEDIA, 512 p.
2. Tomas P. Hebif (2008) “Kozhnye bolezni. Diagnostika i lechenie", perevod s anglijskogo, 3rd edition ["Skin diseases. Diagnosis and treatment", translation from English, 3rd edition].
3. Ficpatrik T., Dzhonson R., Vulf K. “Dermatologiya” (atlas-spravochnik) [["Dermatology” (reference atlas)]/p>
4. Wolff K., Johnson R. (1999) Fitzpatrick's color atlas and synopsis of clinical dermatology.
5. Kubanova A. (ed.) (2015) “Dermatovenerologiya”. Klinicheskie rekomendacii ["["Dermatovenerology". Clinical recommendations]ssijskoe obshchestvo dermatovenerologov.
6. Skripkin YU. (1995) Kozhnye i venericheskie bolezni. Rukovodstvo dlya vrachej.
7. Rodionov A., Zaslavskij D., Sydikov A. (2019) Klinicheskaya dermatologiya. Illustrated rukovodstvo dlya vrachej. Moscow, 712 p.
8. Rodionov A., Zaslavskij D., Sydikov A. (2018) Dermatologiya. Illyustrirovannoe rukovodstvo klinicheskoj diagnostiki po professoru Rodionovu AN [D[Derma[Dermatology. Illustrated guide on clinical diagnosis by professor Rodionov AN], 944 p.
9. Zaslavskij D., Sydikov A., Ivanov A., Nasyrov R. (2020) Venericheskie bolezni i dermatozy anogenital'noj oblasti. Illustrated rukovodstvo dlya vrachej. Moscow, 640 p.
Verrucacid solution for external use approx 2 g fl/pack of cards x1
Trade name: Verrukacid®. INN or group name: phenol, metacresol. Dosage form: solution for external use.
Compound:
Active ingredients: phenol – 588 mg, 3-methylphenol (metacresol) – 392 mg. Excipients: ethyl alcohol (ethanol) 95% - 10 mg, purified water up to 1 g.
Description: mobile oily liquid from pinkish or light yellow to brown with the smell of phenol. Pharmacotherapeutic group: local necrotizing agent. ATX code (D11AF).
Pharmacological properties Has a cauterizing effect, coagulates skin proteins.
Indications for use: Common, filiform and plantar warts, papillomas, genital warts of the skin, dry calluses, keratomas.
Contraindications Increased individual sensitivity to the components of the drug. Pigmented nevi (moles), rashes located on the red border of the lips and mucous membranes. Do not apply the drug to the skin surface area of more than 20 cm2. Children under 7 years old.
Pregnancy and breastfeeding The use of the drug is possible in cases where the expected benefit to the mother outweighs the potential risk to the fetus or child. During lactation, it is not recommended to remove formations located on the mammary glands and hands.
Directions for use and dosage Verrukacid® is intended for external use only.
The drug is applied precisely to the treated area with a special applicator or a small thin wooden stick, preventing it from coming into contact with adjacent healthy areas of skin and mucous membranes. For small papillomas (up to 2 mm in size) and filamentous warts, Verrukacid® is applied once. Larger papillomas and small warts (2-3 mm in size) are smeared with the drug 3-4 times, taking breaks to allow the applied liquid to dry.
Before removing warts with a dense keratinized surface on the hands, plantar warts, keratomas, and dry calluses with Verrucacid®, it is necessary to remove horny layers from their surface. To do this, apply a keratolytic ointment (for example, 10% salicylic or other) for several hours, covering the lubricated area with compress paper or plastic film, and then use a gauze bandage. Possible sealing with adhesive tape. After this, the bandage or adhesive plaster is removed, the skin is steamed in hot water with the addition of soap and soda for 10-15 minutes and the horny layers are removed (cut off with nail scissors or tongs). Verrukacid® is applied to dried skin several times, taking 3-4 minute breaks to allow the drug to dry.
Warts on the hands and soles are treated with Verrukacid® 7-10 times with an interval of 3-4 minutes.
It is enough to treat keratomas and dry calluses 3-4 times with an interval of 3-4 minutes.
If it is necessary to apply the drug repeatedly, in order to avoid burns to the surrounding skin, it is advisable to lubricate it with zinc paste. The paste is removed with a dry gauze swab after the last portion of Verrukacid® has dried.
It is not recommended to remove genital warts yourself; they are treated with Verrucacid® in the treatment room of the clinic by a dermatovenereologist or urologist. The drug Verrukacid® is applied to each element separately 1-2 times with an interval of 3-4 minutes.
Repeated treatment, if necessary, is carried out 6-8 days after the crust falls off. 4-5 procedures are allowed.
Side effects Burn (in case of contact with healthy skin). If the drug accidentally comes into contact with healthy skin, it is necessary to immediately, carefully, without rubbing, remove it from the skin, and then treat the affected areas with 10-40% ethyl alcohol or alcohol-containing liquids (vodka, lotion, cologne) and wash thoroughly with warm water and soap. If a burn occurs, it is recommended to use anti-burn and healing agents.
Swelling and redness of the skin when applied to the eye area, which goes away on its own.
Allergic reactions.
Overdose Not detected.
Interaction with other drugs The components of the drug easily dissolve in the ointment base, and therefore it is not recommended to lubricate the skin area treated with Verrucacid® with any ointments.
Special instructions Do not allow the drug to come into contact with mucous membranes, especially the mucous membrane of the eyes. In case of contact, immediately rinse eyes with plenty of water and consult an ophthalmologist.
It is unacceptable to bandage the areas treated with the drug, seal them with a plaster, lubricate them with any ointments and remove the crust; you cannot reapply the drug before the time limits specified above. Clothing made of synthetic fabrics should not touch the area of skin treated with the drug.
It is not recommended to apply Verrucacid® to formations located in skin folds (inguinal folds, anal area, interdigital spaces, etc.) and heavily sweating areas in order to avoid burns to unaffected skin of the contacting surface or as a result of the drug spreading over wet skin.
The area of skin treated with Verrucacid® must be air-dried; it must not be lubricated with any ointments or washed with water on the first day after treatment.
When used correctly, the drug does not leave scars.
Particular care and caution is required when treating children with the drug.
Release form Solution for external use in dark glass bottles with and without applicator, 2 and 10 g.
Each bottle, along with instructions for medical use, is placed in a cardboard pack.
Additionally, each cardboard pack can include a plastic lid with an applicator.
Storage conditions In a place protected from light at a temperature of 15 to 25 ° C.
Keep out of the reach of children.
Shelf life: 5 years. Do not use after expiration date.
Conditions for dispensing from pharmacies Dispense without a prescription.