Heart valve prolapse is a disease accompanied by a malfunction of the valve located between the ventricle and the left atrium and is a common and relatively harmless anomaly, during which an unnatural protrusion of the valve leaflets appears when the heart contracts.
A heart valve is a movable valve consisting of individual elements that blocks the openings for blood circulation from one part of the heart to another. The valves' job is to control blood flow. There are four valves in the heart - mitral, tricuspid, aortic and pulmonary, which allow blood to flow in one direction and prevent it from returning back. When the heart muscle contracts, pressure is created, and blood is ejected from the heart, and the valves that regulate the movement of blood in this direction at the time of muscle contraction open. After the muscle contracts, the pressure in the heart drops, the valve closes, and blood cannot return to the heart. Among other types of prolapse, the most common is mitral valve prolapse, which is caused by congenital weakness of the connective tissue that makes up the heart valves.
Definition of rectal prolapse
Rectal prolapse occurs as a result of a violation of its fixation with the walls of the pelvis when the pelvic floor muscles are weakened, which is approximately 9% of all diseases of the colon. Rectal prolapse (rectal prolapse) is a telescopic protrusion of the rectum through the anus; intussusception is a condition in which the telescopic displacement of the rectum does not extend beyond the anal canal. Both of these diseases lead to discomfort and minor pain (rarely) in the perineal area. Sometimes rectal prolapse is accompanied by ischemia and necrosis of the wall, which requires emergency surgery.
What is genital prolapse
Pelvic organ prolapse occurs in stages, the uterus gradually shifts towards the exit from the vagina along with adjacent organs. The vagina will gradually change shape - either one wall (front or back) or both will sag. If no measures are taken, then over time the uterus may completely fall out, which will significantly disrupt the quality of life.
Dear women, do not bring yourself to a state where nothing can be done - only surgery and removal of the uterus! The price of seeing a gynecologist is nothing compared to the value of your health.
Etiology and pathogenesis of rectal prolapse (rectal prolapse).
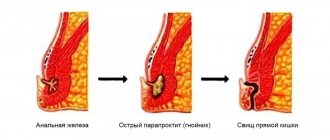
Many theories of the occurrence of rectal prolapse have been described. About 100 years ago, Moschcowitz presented rectal prolapse as a sliding hernia through a defect in the pelvic fascia. Broden and Snellman later showed on cinedefecography that rectal prolapse is an intussusception of the rectum. Rectal prolapse is more common in women than men and is likely associated with childbirth. Prolonged straining during pushing, anatomical features (wide pelvis) are producing and predisposing factors in the occurrence of this disease. In women, the incidence of this disorder increases with age, while men are more often affected at a young age (20-40 years) and they almost always have a history of pre-existing rectal disease, such as anal atresia, or surgical treatment of diseases of the rectum and anal canal. In women, the pudendal nerves may be damaged during childbirth, leading to pelvic floor dysfunction such as urinary and fecal incontinence or genital or rectal prolapse.
Mitral valve prolapse: clinical variants, modern concepts
Despite more than half a century of history of studying mitral valve prolapse (MVP), a number of issues related to diagnosis, determination of prognosis, and tactics for managing patients with this pathology continue to cause difficulties for practitioners. This is largely due to the morphological and clinical heterogeneity of this condition, suggesting the existence of different variants of mitral prolapse.
MVP is a syndrome inherent in various nosological forms and each time requiring nosological verification. The list of diseases manifested by MVP is extensive, which is explained by the complexity of the structure of the heart valve apparatus and the variety of mechanisms of mitral prolapse. MVP may be based on changes in the fibrous ring, mitral valve leaflets and chordae attached to them, dysfunction of the papillary muscles, and impaired contractility of the left ventricular myocardium.
In clinical practice, MVP can appear in five forms:
- primary MVP;
- secondary MVP as a consequence of myocardial diseases;
- secondary MVP as a manifestation of hereditary monogenic syndromes;
- mitral prolapse as a variant of the norm or a manifestation of a minor anomaly of cardiac development;
- MVP as an “echocardiographic disease”.
MVP as an “echocardiographic disease”
MVP as an “echocardiographic disease” is a situation of erroneous diagnosis of mitral prolapse. At the dawn of the introduction of two-dimensional echocardiography into clinical practice, MVP was diagnosed in 5–15% and even 35% of people examined. Such significant overdiagnosis was associated with a misconception about the flat configuration of the mitral valve. Ultrasound series from the late 1980s. demonstrated the three-dimensional (saddle-shaped) shape of the mitral valve ring and made assessment from a parasternal longitudinal position mandatory. The modern definition of MVP interprets it as systolic bulging of one or both mitral valve leaflets at least 2 mm above the plane of the mitral annulus with mandatory registration along the long axis of the heart. As can be seen, the definition clearly specifies both the anatomical criteria and the technical aspects of the research procedure.
The transition to unified criteria for ultrasound diagnostics and the publication of the results of the Framingham study made it possible to eliminate contradictions in views on the prevalence of MVP, which turned out to be significantly lower than previously thought. Of the 3491 participants in the Framingham Study who underwent 2D echocardiography using consensus diagnostic criteria, 47 (1.3%) had classic (with mitral leaflet thickening) and 37 (1.1%) nonclassical MVP, with an overall incidence of 2.4% [ 1]. Similar results were obtained based on the Russian population.
Secondary MVP in myocardial diseases
Among other reasons, MVP can develop against the background of coronary pathology, rheumatism, cardiomyopathy, myocarditis, myocardial dystrophy - conditions that cause impaired contractility of the left ventricular myocardium of a local or diffuse nature, dysfunction or separation of the papillary muscles. Such variants of mitral prolapse are considered secondary, since they are part of the structure of the clinical picture of the corresponding disease. The presence of secondary MVP in the absence of significant mitral regurgitation has little effect on the symptoms of the underlying disease. The exception is cases of acute mitral regurgitation that develops as a result of avulsion of the papillary muscle during myocardial infarction or blunt cardiac trauma.
Primary MVP
Primary MVP is the only variant of mitral prolapse that claims to be nosologically independent. The origin of primary MVP is associated with the pathology of the mitral valve leaflets, caused by a specific cause - mesenchymal inferiority as part of hereditary disorders (dysplasias) of connective tissue (HCT). NNCT is a group of genetically heterogeneous and clinically polymorphic pathological conditions, united by a violation of the formation of connective tissue in the embryonic and postnatal periods. The genetic heterogeneity of NNCT implies both a monogenic and multifactorial nature of the disease, and clinical polymorphism is associated with the ubiquity of connective tissue in the body and the variety of manifestations of congenital “weakness” of its individual components.
In accordance with modern concepts, two categories of NNCT are distinguished: classified (having agreed upon diagnostic recommendations) and unclassified (also known as dysplastic phenotypes). Consensus diagnostic recommendations include monogenic syndromes caused by mutations in the genes of extracellular matrix proteins, growth factor receptors, and matrix metalloproteinases. The most famous and clinically significant of them are Marfan and Ehlers-Danlos syndromes, MASS phenotype, joint hypermobility syndrome and others. The classified syndromes also include primary MVP. To date, three gene loci responsible for its occurrence have been found, located on the 11th, 13th and 16th chromosomes. The search for genes involved in the development of mitral prolapse continues; it is assumed that their identification will create the prerequisites for screening asymptomatic patients at risk of developing mitral regurgitation. However, despite all the attractiveness of molecular genetic research methods, one cannot help but note their inaccessibility in everyday practice. It is no coincidence that existing recommendations for NNCT give priority in the diagnosis of primary MVP to a combination of clinical and ultrasound data.
The morphological reflection of congenital “weakness” of connective tissue and a marker of primary MVP is the so-called myxomatous degeneration of the mitral valve leaflets. It is characterized by proliferation of the middle - spongy layer of the valve with excessive accumulation of glycosaminoglycans and disorganization of collagen fibrils (Fig. 1). In this case, the leaflet thickens, changes its mechanical properties, loses its ability to withstand pressure in the cavity of the left ventricle during systole and prolapses.
The clinical picture of primary MVP is varied; it can be either asymptomatic or clinically manifest. Symptoms of primary MVP are represented by a combination of the following syndromes:
- disturbances of intracardiac and general hemodynamics;
- manifestations of vegetative-vascular dystonia;
- other (extravalvular) manifestations of “weakness” of connective tissue.
It is this triad that determines the clinical uniqueness of primary MVP, distinguishing it from other variants of mitral prolapse. The degree of expression of each of these components may be different, which determines the significant clinical polymorphism inherent in primary MVP.
The main method and “gold standard” for diagnosing MVP is two-dimensional echocardiography. It allows you to establish the fact of mitral prolapse, assess the thickness of the mitral leaflets, and determine the degree of mitral regurgitation. These characteristics are extremely important in understanding the severity of the patient’s condition, prognosis and management tactics. Thus, an increase in the thickness of the valve by more than 5 mm (with a norm of 2–4 mm) is reliable evidence of its myxomatous degeneration, which is a morphological substrate and the main marker of primary MVP. Depending on the thickness of the sash, classic (with a sash thickness of 5 mm or more) and non-classical (less than 5 mm) PMC are distinguished. Since the outcomes of MVP are determined by the degree of disturbance of intracardiac hemodynamics, an equally important parameter is the severity of mitral regurgitation, assessed during a Doppler ultrasound study.
Autonomic dysfunction is recognized as the leading mechanism explaining the diverse symptoms of primary MVP, but the presence of asymptomatic patients does not allow us to unambiguously determine its pathogenetic role: whether it is the cause of MVP or a random combination. Nevertheless, most researchers consider changes in autonomic homeostasis to be an obligatory attribute of MVP. It is autonomic dysfunction that explains such common manifestations of “MVP syndrome” as cardialgia, most cardiac arrhythmias, instability of blood pressure, lipothymia, hyperventilation and asthenic syndromes. To explain the prevalence of autonomic dysfunction in MVP, many hypotheses have been proposed, including congenital changes in the perinervia, a systemic defect in biological membranes, perinatal damage to the hypothalamic structures, and finally, the recently actively discussed version of the pathogenetic role of magnesium deficiency.
But no matter how vivid the manifestations of autonomic dysfunction are, the basis of hemodynamic disturbances in MVP is still mitral regurgitation. Its inevitable consequence is volume overload and dilatation of the left heart, which leads to atrial fibrillation and progression of heart failure. Complications also include thromboembolism from myxomatically altered valves and the possibility of developing secondary infective endocarditis.
The cause of adverse consequences and complications of primary MVP is not only the valve mechanism. As has recently been shown, disturbances in general hemodynamics in this pathology occur not only through mitral regurgitation, but also through defects in the structure and function of the extracellular matrix of the myocardium, which are a consequence of the congenital “weakness” of connective tissue. These defects can cause diastolic dysfunction, decreased myocardial contractility and the development of secondary cardiomyopathy.
Recently, ideas about the severity of the consequences of MVP have been reassessed. Previously, MVP was considered a pathology with frequent and serious complications (including stroke, atrial fibrillation, heart failure) and a high need for surgical correction of mitral regurgitation. Contrary to previous knowledge, the results of the Framingham study gave reason to consider MVP as a benign condition with a low probability of adverse outcomes. In particular, the incidence of atrial fibrillation, cerebral stroke, and syncope in patients with MVP turned out to be comparable to similar outcomes in individuals with intact valves [1]. The question of risk stratification in MVP has become urgent. To date, pathological variants associated with high risk and poor prognosis have been identified. The likelihood of hemodynamic disorders increases with a high degree of mitral regurgitation and a leaflet thickness of more than 5 mm, indicating its myxomatous degeneration.
When discussing possible complications and outcomes of mitral prolapse, it is necessary to emphasize that primary MVP has a progressive course. Although it is a congenital pathology, it does not occur in newborns, is characterized by low incidence rates among children and young people, and affects mainly mature patients. The time interval preceding the clinical manifestation of MVP is the period during which an increase in myxomatous changes in the valve leaflet occurs up to its deformation to a degree that impairs the closure function and leads to mitral regurgitation. The appearance of the latter radically changes the well-being and fate of patients, which is clearly reflected in Fig. 2, which correlates the staged course of MVP with the likelihood of complications. Moreover, if thromboembolism, infective endocarditis, and “functional” arrhythmias are recorded with approximately the same frequency in different age periods, then the frequency of congestive heart failure and atrial fibrillation soars after 50 years.
The role of primary MVP in circulatory decompensation and the occurrence of atrial fibrillation in adulthood and old age is often underestimated. The manifestation of valvular pathology in elderly patients often becomes the cause of diagnostic errors. Practitioners are poorly aware of NNCT and, according to the prevailing stereotype, attribute circulatory failure and heart rhythm disturbances in people in the second half of life almost exclusively to coronary pathology. Often they are not embarrassed by either the absence of angina or scarring ECG changes, or the presence of a rough systolic murmur, reflecting the presence of mitral regurgitation.
A separate component of the clinical polymorphism of primary MVP are external and visceral markers of “weakness” of connective tissue. Since the connective tissue defect underlying the increased compliance of the valve leaflets is generalized, signs of mesenchymal inferiority are also determined from the skin, musculoskeletal system, and internal organs. The currently known visceral markers, as well as their most important clinical consequences and outcomes, are given in Table.
Since signs of systemic involvement of connective tissue are indirect confirmation that MVP belongs to NNCT (i.e., evidence of its “primary”), their identification is important from the point of view of diagnosis and assessment of prognosis. Thus, the likelihood of clinically and hemodynamically significant MVP is extremely low in individuals who do not have these signs. In order to unify approaches to assessing the systemic involvement of connective tissue, in particular, determining the threshold of stigmatization and structuring signs with highlighting the most informative, it is proposed to use the Ghent criteria (Ghent nosology, 2010) accepted by the world community for Marfan syndrome.
Recent times have been characterized by the emergence of new ideas about the cellular and molecular mechanisms of primary MVP. The leading role in the mechanisms of primary MVP is played by transforming growth factor β (TGF-β), a protein that activates the growth of fibroblasts and regulates the formation and degradation of the extracellular matrix. It was found that a number of manifestations of NNCT are caused by changes in the activity of TGF-β. In particular, increased expression of TGF-β was found in myxomatous valves. There is a natural desire to inhibit the increased activity of TGF-β in order to prevent the progression of myxomatosis, and such tools are already known - these are neutralizing antibodies (in experiment), angiotensin II receptor blockers and β-blockers (in experiment and clinic). The use of these agents opens up new ways to correct the consequences of impaired mesenchymal insufficiency and, perhaps, will serve as an alternative to surgical treatment.
However, the real possibilities of pathogenetic therapy for primary MVP still remain modest. Among the treatment methods relatively widely introduced into clinical practice, the use of magnesium preparations should be highlighted. The pathogenetic rationale for their use is the idea of primary MVP as a clinical form of genetically determined magnesium deficiency. It is known that under conditions of a lack of magnesium ions, the synthesis of connective tissue proteins slows down, and the activity of enzymes involved in the destruction of collagen and elastin, on the contrary, increases. In other words, under conditions of magnesium deficiency, connective tissue is destroyed faster than it is synthesized.
A number of studies have shown the fundamental possibility of eliminating the characteristic cardiac symptoms and ultrasound manifestations of MVP under the influence of magnesium preparations. One of the most famous of them belongs to a team of domestic authors under the leadership of Academician A.I. Martynov. A 6-month course of therapy with Magnerot at a dose of 3 g per day led to a decrease in the depth of prolapse and the degree of myxomatous degeneration of the valve leaflets. Along with this, a reduction in the clinical symptoms inherent in this category of patients was achieved [5]. Over the past time, many studies of similar design have been carried out, in which similar results were obtained.
Along with magnesium preparations, vitamins, other micro- and macroelements, and anabolics—drugs related to the metabolism of connective tissue and capable of influencing the biochemical mechanisms underlying myxomatous degeneration of the valve and its prolapse—may also be candidates for the role of means of pathogenetic treatment of primary MVP. All of them, in one or another combination, find use in the complex therapy of primary MVP.
When discussing the treatment of MVP, it is necessary to clearly understand that hemodynamically significant (accompanied by signs of heart failure) prolapse is a heart defect that requires surgical correction. Such patients should be promptly referred to a cardiac surgeon to resolve the issue of mitral valve replacement or plastic surgery. Commenting on the indications for surgical treatment of mitral regurgitation, we note that the current AHA/ACC2014 recommendations do not confirm the advisability of surgical treatment only for patients with preserved ejection fraction and left ventricular size.
Secondary MVP in syndromic NNCT
MVP can occur with monogenic connective tissue defects, such as Marfan, Loeys–Dietz, Ehlers–Danlos syndromes, osteogenesis imperfecta, and pseudoxanthoma elasticum. It accounts for 0.25–2% of cases of mitral prolapse. Among all monogenic syndromes, MVP is most often observed in Marfan syndrome - 75% of cases (and more severe variants with valvular myxomatosis - 28%) [6]. The prevalence of MVP in patients with Ehlers–Danlos syndrome is significantly lower—6% [7]. PMC in monogenic NNCT is classified as secondary, since it is part of the structure of the clinical and morphological manifestations of the corresponding pathology. However, it is characterized by the same myxomatous changes in the valves as primary MVP, and in this way it is similar to it.
MVP as a variant of the norm or a manifestation of a minor anomaly of cardiac development
The borderline degree of prolapse, the absence of myxomatous thickening of the leaflets, significant mitral regurgitation and family history allow us to consider MVP as a normal variant, a transient age-dependent phenomenon or a minor cardiac anomaly. It is these cases of mitral prolapse that most often occur in clinical practice, especially in adolescents, slender boys, girls and young women, creating the impression that MVP is extremely common in the population.
Among the causes of such “innocent” cases of MVP are congenital microanomalies of the architecture of the mitral complex, asynergy of contraction and relaxation of the myocardium, disruption of valvular innervation, etc. Separately, it should be noted cases of shallow, asymptomatic prolapse that occurs with age, arising in the puberty period due to the uneven development of individual elements of the valve mitral complex and their incomplete functional correspondence to each other. In this case, the area of the chordal-valve apparatus turns out to be excessive, as if stored for future use. At the end of puberty, as the volume and mass of the left ventricular myocardium increases, this discrepancy is often leveled out (and in women to a lesser extent than in men, which obviously explains the predominance of women among patients with MVP).
It is necessary to be aware that in the absence of data from a molecular genetic study, it is not possible to reliably exclude the preclinical stage of primary MVP in all these cases, which justifies recommendations for dynamic monitoring of such patients.
Half a century has passed since the first description of MVP by J. V. Barlow, who established the connection between late systolic murmur and mitral regurgitation. What results have we reached during this time, what conclusions can we draw?
- MVP is a syndrome inherent in different nosological forms and in each case requires the establishment of a nosological diagnosis.
- Clear diagnostic criteria for MVP have been established that should be followed.
- The ambiguity of the prognosis for MVP dictates the need for stratification of risk factors.
- Despite the capabilities of molecular genetic research methods, the diagnosis of PMC continues to be a synthesis of clinical and ultrasound manifestations with an assessment of signs of systemic involvement of connective tissue.
- Advances in understanding the mechanisms of development of myxomatosis open up new prospects for the treatment of primary MVP.
In conclusion, let us recall that both the All-Russian Scientific Society of Therapists and the Russian Society of Cardiology have issued a series of recommendations devoted to the diagnosis and treatment of NNST and MVP. By referring to these documents, a practitioner can clarify details that were not sufficiently covered in this article.
Literature
- Freed LA, Levy D, Levine RA et al. Prevalence and clinical outcome of mitral-valve prolapse // N. Engl. J. Med. 1999; 341 (1): p. 1–7.
- Sainger R., Grau J.B., Branchetti E. et al. Human myxomatous mitral valve prolapse: role of bone protein 4 in valvular interstitial cell activation // J. Cell. Physiol. 2012; 227 (6): p. 2595–2604.
- Boudoulas KD, Boudoulas H. Floppy mitral valve (FMV)/mitral valve prolapse (MVP) and the FMV/MVP syndrome: pathophysiologic mechanisms and pathogenesis of symptoms // Cardiology. 2013; 126 (2): p. 69–80.
- Delling FN, Vasan RS Epidemiology and pathophysiology of mitral valve prolapse: new insights into disease progression, genetics and molecular basis // Circulation. 2014; 129 (21): p. 2158–2170.
- Martynov A.I., Akatova E.V., Nikolin O.P. Results of long-term therapy with magnesium orotate in patients with mitral valve prolapse // Cardiovascular Therapy and Prevention. 2012; 11 (3): p. 30–35.
- Taub CC, Stoler JM, Perez-Sanz T. et al. Mitral valve prolapse in Marfan syndrome: an old topic revisited // Echocardiography. 2009; 26 (4): p. 357–364.
- Dolan AL, Mishra MB, Chambers JB, Grahame R. Clinical and echocardiographic survey of the Ehlers-Danlos syndrome // Br. J. Rheumatol. 1997; 36 (4): p. 459–462.
A. V. Klemenov, Doctor of Medical Sciences
GBUZ NO City Clinical Hospital No. 30, Nizhny Novgorod
Contact Information
DOI: 10.26295/OS.2019.64.93.014
Mitral valve prolapse: clinical options, modern concepts / A. V. Klemenov For citation: Attending physician No. 9/2019; Page numbers in the issue: 65-69 Tags: heart, mitral prolapse, congenital mesenchymal inferiority
Symptoms of rectal prolapse
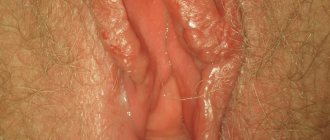
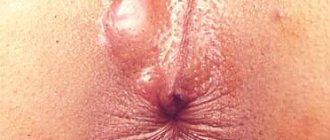
Most patients with rectal prolapse come to the surgeon with complaints about the prolapse itself. They often describe the sensation of “something coming out,” especially during bowel movements (Figure 1). Many people complain of feeling like they are “sitting on a ball” until spontaneous or manual reduction pushes the prolapsed bowel back inside. Very often this disease is associated with soiling of underwear and the sensation of mucus secretion from the rectum. With prolapse, dysfunction of the colon often occurs, such as constipation and straining to defecate, as well as fecal incontinence. When colon function is impaired, damage to the mucous membrane (ulcers, erosions, etc.) often occurs.
Fig1. Prolapse of all rectal walls
Classification
Diagnosed cases of the disease in adolescents and adults are divided into two types:
- MVP with a high risk of developing regurgitation;
- moderate prolapse with a low risk of developing minor regurgitation.
Doctors can also divide the disease into stages:
- moderate prolapse 0-1 degree: also known as primary (even during pregnancy, no negative symptoms are felt; the body independently regulates the additional load on the heart);
- MVP stage 2 (transitional): signs - intracardiac pressure increases, in adolescents and during pregnancy there is constant shortness of breath and fatigue;
- 3rd (decompensated stage): significant damage to the heart muscle can be diagnosed.
Diagnosis of rectal prolapse (rectal prolapse)
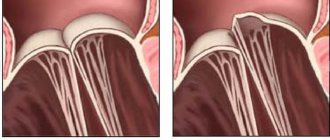
The diagnosis of spontaneous prolapse is obvious - prolapse of all layers of the intestine looks like a prolapsed cylinder with concentric folds.
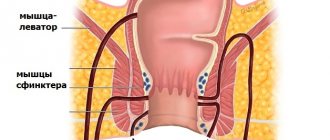
In some patients, straining may be required to diagnose it. In these cases, prolapse is best detected in a squatting position or on a chair. A number of patients come to a consultation with a surgeon with photographs of their prolapsed intestine already taken. It is very important that before choosing one or another method of correcting rectal prolapse, it is necessary to make a differential diagnosis between prolapse of all parts of the rectal wall and prolapse of only its mucous membrane. Prolapse of all layers looks like a prolapsed cylinder with concentric folds and grooves, in contrast to prolapse of only the mucosa, where these folds and grooves have a strictly radial orientation. During the examination, the doctor should pay attention to maceration and erosion of the perianal skin. A thorough digital examination of the rectum is very important to identify concomitant pathology of the anal canal and to assess the adequacy of sphincter muscle tone at rest, during compression, as well as the function of the puborectal muscle.
Next, you should definitely perform a colonoscopy, or flexible sigmoidoscopy, followed by irrigoscopy with double contrast to exclude or identify any abnormalities of the colon mucosa.
Defecography
As a rule, it is not mandatory when examining prolapse of all the walls of the rectum, because it is visually obvious, however, this study can be the main diagnostic component when examining for internal invagination of the rectum (hidden prolapse) or as part of a system for examining the pelvic floor muscles.
Anal manometry
helps in assessing sphincter function because chronic rectal prolapse typically affects the internal sphincter, resulting in decreased resting pressure at its projection. In manometric studies of patients with rectal prolapse, some authors report an abnormality or complete absence of the anorectal inhibitory reflex, and abnormally low pressure in the projection of the sphincters at rest is also noted, while at the same time the pressure during sphincter contraction was normal.
Anal electromyography
and the study of the speed of excitation along the fibers of the pudendal nerve are generally not clinically important studies for this disease, unless in the history of the disease there is a need for particularly strong straining for the act of defecation. In such cases, anal electromyography can be used to investigate the presence of inadequate or paradoxical (perverted) contraction of the puborectalis muscle - anism (non-relaxing puborectalis muscle or spastic pelvic floor).
Colon transit time study
should be performed in patients with a medical history indicating constipation, otherwise the wrong surgical method may be chosen.
Symptoms of genital prolapse
In the first stages, the disease does not manifest itself in any way, and minor deviations can only be detected during a gynecological examination. Later, nagging pain occurs in the lower abdomen, in the lumbar region, which intensifies and radiates into the vagina when moving. In addition, you may see:
- white discharge, odorless, similar in consistency to mucus;
- discomfort when emptying or straining the abdominal muscles;
- inability to hold urine;
- pain and bleeding after sexual intercourse;
- frequent constipation, gas incontinence;
- at the exit from the vagina, a soft, mobile formation (sagging wall) can be felt.
Treatment of rectal prolapse (prolapse of the rectum)
Obviously, conservative measures are not able to eliminate pelvic organ prolapse, but they can slow down its progression, help correct certain problems (intestinal dysfunction, urinary incontinence), and also increase the effectiveness of surgical treatment.
Rectal prolapse is almost always treated surgically; with rectoanal intussusception, every therapeutic effort should be made to avoid surgery.
It is worth noting that in the presence of inadequate or paradoxical (perverted) contraction of the puborectal muscle (anism), a feedback training system or the administration of Botulinum toxin can be used.
Only surgical intervention can restore the structure and function of the pelvic floor, as well as significantly improve the quality of life of patients with rectal prolapse.
Surgical treatment of rectal prolapse (rectal prolapse)
Watch a video of operations performed by Professor K.V. Puchkov. You can visit the website “Video of operations of the best surgeons in the world.”
A large number of different methods have been proposed for the treatment of rectal prolapse. The surgeon must determine which surgical method will be best for each patient. In this case, a large number of individual factors and features of surgical methods are taken into account.
The main individual factors are the patient's gender and age, the patient's general medical condition, colon function, and the presence or absence of fecal incontinence.
It is imperative to take into account the length of the prolapsed section of the colon, the effect of the operation on colon function and continuity, the risk of complications during the operation, the frequency of relapses with a particular type of operation, and the individual experience of the surgeon.
Before choosing one or another method of correcting rectal prolapse, it is necessary to make a differential diagnosis between prolapse of all parts of the rectal wall and prolapse of only its mucous membrane. Prolapse of all layers looks like a prolapsed cylinder with concentric folds and grooves, in contrast to prolapse of only the mucosa, where these folds and grooves have a strictly radial orientation.
Surgical approaches in the treatment of rectal prolapse
There are two main approaches to surgery for rectal prolapse – abdominal surgery and perineal surgery.
The most common abdominal surgery for rectal prolapse is rectal fixation (rectopexy).
, performed in isolation or synchronously with
resection of the sigmoid colon.
A typical perineal operation is perineal rectosigmoidectomy (Altmayer procedure) or resection of the mucosal sleeve of the prolapsed rectum (DeLorme procedure).
The specificity of each operation must be determined taking into account the condition and the totality of all pathological changes in each patient, but some generalizations can be made. Elderly patients with a high degree of surgical and anesthetic risk are best treated with perineal surgery, which can be performed under regional anesthesia or even local anesthesia with intravenous sedation. Healthy patients with normal colon function can easily undergo both abdominal rectopexy with or without synchronous resection of the sigmoid colon, and perineal resection of the rectum and sigmoid colon, with or without synchronous levatoroplasty for fecal incontinence
.
Colon function plays a role in the choice of surgical plan. Patients with constipation should usually undergo rectopexy and sigmoid resection, whereas patients with fecal incontinence and rectal prolapse should undergo simultaneous abdominal rectopexy or perineal resection of the rectum and sigmoid colon along with levatoroplasty. A recurrent case of the disease requires information about the type of first operation, since this information will determine further treatment tactics; the first operation may limit the choice of surgery in case of relapse due to changes in the blood supply to the operated rectum.
Unfortunately, there is a lack of high-quality data regarding which method is optimal for treating rectal prolapse. In 2008, a comprehensive analysis of randomized trials was attempted and found that there was insufficient data for analysis, although some conclusions were drawn. The method of fixation of the intestine during rectopexy does not affect the result. Transection of the collateral ligaments of the rectum resulted in a high incidence of constipation, whereas resection and rectopexy resulted in less constipation. Finally, laparoscopic procedures resulted in faster postoperative recovery and fewer complications.
Laparoscopic approach in the treatment of rectal prolapse
In recent years, the popularity of the laparoscopic approach in colorectal surgery has increased, and in many cases the use of this approach is justified. The laparoscopic approach for the treatment of prolapse of all layers of the rectal wall has been used in multiple techniques for abdominal correction of rectal prolapse, such as rectopexy, resection with rectopexy, and mesh surgery
. In general, the success and complications are comparable to traditional surgeries with one advantage - shorter hospitalization and faster recovery.
The results of 10 years of experience with laparoscopically assisted rectopexy with bowel resection in a single institute in 117 patients with rectal prolapse are reported. Mortality was 0.8% (1 patient), and complications after surgery developed in 9% of patients (10 people out of 117); 77 of 117 patients (66%) were followed for an average of approximately 62 months. Recurrence of full-wall rectal prolapse occurred in 2.5% of cases, prolapse of the rectal mucosa in 18%. Other authors examined 77 patients who underwent laparoscopic posterior mesh rectopexy (modified Wells procedure). They described only one case of conversion to open surgery due to severe adhesions and 2 intraoperative complications, which were easily corrected during laparoscopic surgery. There was a relapse of the disease in only one patient, and 90% of patients during long-term follow-up (more than 34 months) were satisfied with the results of the operation.
Treatment
Laser rejuvenation
At the initial signs of vaginal and uterine prolapse, non-invasive treatment is prescribed - laser lifting of the vaginal walls. At the Marina Ryabts clinic, a Fotona laser unit (Slovenia) is used for the treatment and prevention of genital prolapse. This is a top laser, widely used by gynecologists to solve functional and aesthetic problems. The quality of treatment will be high, and the result will be long-lasting and predictable if you complete the course of procedures. Even one session will make you feel better, but to consolidate the effect, you need to come to the procedure three times. The advantages of the laser method are painlessness, minimal rehabilitation (sexual activity, for example, can be resumed 3 days after the session), no trauma to the vaginal walls (the laser affects deeper layers without damaging the surface of the mucous membrane). Laser treatment of pelvic organ prolapse is widely used all over the world, is not contraindicated for patients with a history of cancer and is perfectly combined with other gynecological “cosmetology” procedures: biorevitalization, contour plastic surgery.
Surgical correction
“Advanced” prolapse, accompanied by hernias, is treated with the traditional surgical method. Rehabilitation is carried out under general anesthesia, followed by rehabilitation with a number of restrictions. So, for a long time you will not be able to visit the sauna and swimming pool, swim in open water, have sex, or take a bath.
Recurrence of rectal prolapse
Although rectal prolapse has historically had a high recurrence rate (up to 50%), current data suggest that recurrence of rectal prolapse after rectopexy with sigmoid colon resection is less than 10%. In general, perineal surgery for rectal prolapse has a higher risk of recurrence than abdominal surgery.
Patients with recurrent rectal prolapse need examination - manometry and defecography . It is very important to inform the patient that even if the prolapse is corrected, there are still concomitant dysfunctions of the colon (constipation or diarrhea) that are not corrected by rectopexy. One of the most important points in choosing the best surgical option for correcting recurrent rectal prolapse is assessing the remaining blood flow after the first operation. The first operation performed for rectal prolapse plays a major role in determining the type of intervention to correct recurrent prolapse, since bowel resection interrupts the blood supply to the colon. Any patient who has undergone a primary resection of the rectum or sigmoid colon with anastomosis requires a very careful examination before undergoing a second operation, including a careful study of the protocol of the first operation.
The obvious risk of repeat resection is ischemia of the segment of colon between the two anastomoses. For example, if a patient first underwent a perineal rectosigmoidectomy, then a repeat perineal rectosigmoidectomy or transabdominal rectopexy may be performed. However, in such cases, transabdominal rectopexy with resection of the sigmoid colon should be excluded due to the risk of ischemia of the remaining rectal segment. For those patients who initially underwent transabdominal rectopexy but experienced a relapse, repeat transabdominal rectopexy is the procedure of choice. Recurrence of full-wall rectal prolapse can be successfully corrected with the same type of surgery that was performed initially.
Reports in the literature indicate a successful outcome of correction of recurrent rectal prolapse in 85-100% of cases. Unfortunately, while most authors justify the initial surgical technique, for repeat procedures they do not attempt to adequately justify the choice of type of operation. For these reasons, there appears to be insufficient data on which to make an intellectually informed treatment decision for the correction of recurrent rectal prolapse. There is no specific algorithm available for use in selecting the best surgery to treat recurrent rectal prolapse, except that most reports suggest that young patients should be treated with a transabdominal approach and older patients with a perineal approach.
A large retrospective study was conducted on 78 patients with recurrent rectal prolapse selected from a group of 685 patients who underwent primary correction of rectal prolapse. When studying recurrence after the second and sometimes even 3 operations, they showed that the approach (abdominal versus perineal) determines the frequency of recurrence of the disease. Abdominal surgery was associated with lower recurrence of rectal prolapse. The authors concluded that, if possible, the abdominal approach should be used to correct recurrent rectal prolapse.
How to diagnose?
The most effective way to determine the presence of the disease is to do an ultrasound of the heart in Rostov-on-Don. Using an ultrasound examination, the doctor will gain an understanding of the degree of prolapse of the valves and the volume of regurgitation.
For diagnosis, echocardiography is also performed, which reveals:
- Stage of prolapse;
- Connective tissue dysplasia;
- The amount of blood flowing back into the atrium.
According to the study, the doctor determines the degree of regurgitation of the mitral valve, i.e. at what level does it occur?
- I – valves;
- II – reaches the middle of the left atrium;
- III - reaches the opposite end of the atrium.
List of published works on the topic “Rectal and genital prolapse”
“Minimally invasive colon surgery”, K. V. Puchkov, D. A. Khubezov
- Puchkov K.V., Karpov O.E., Filimonov V.B. Laparoscopic rectopexy // Ros. magazine gastroenterology, hepatology, coloproctology.-1997.-T. 7, No. 5 (add. 4). -P.248.
- Puchkov K., Filimonov V., Titov G., Chubezov D. Laparoscopic technology in coloproctology // Proktologia. – 2001. – Suppl. No. 1. – P. 97.
- Puchkov K.V., Khubezov D.A. Laparoscopic rectopexy // Problems of coloproctology. Vol. 18. - M., 2002. - P. 194-195.
- Puchkov K.V., Khubezov D.A., Yudina E.A.. Laparoscopic one-stage recto- and sacrovaginopexy // Current issues of coloproctology: abstract. report 1st Congress of Coloproctologists of Russia with international. participation / edited by G.I. Vorobyova, G.P. Kotelnikova, B.N. Zhukova.- Samara: State Enterprise <Perspective>: SamSMU, 2003. - P. 398-399.
- Puchkov K.V., Khubezov D.A., Politova A.K. Laparoscopic recto- and sacrovaginopexy in patients with a combination of rectal prolapse and uterine prolapse // Current problems of modern surgery: tr. congress, Moscow, February 22-25. 2003 – M., 2003. – P.36.
- Puchkov K.V., Khubezov D.A., Yudina E.A., Khubezov A.T., Podyablonsky A.V. Laparoscopic recto- and vaginosacropexy with one implant // Current problems of pelvic surgery. - M., 2003.- P. 79 –81.
- Puchkov K.V., Ivanov V.V., Bakov V.S., Usachev I.A. Optimization of techniques for surgical treatment of pelvic prolapse // Minimally invasive technologies in surgery: materials from interregion. scientific-practical conf. - Makhachkala: IPC DSMA, 2005. - P.159-160.
- Puchkov K.V., Khubezov D.A. Minimally invasive colon surgery: A guide for doctors. - M.: OJSC "Publishing House "Medicine", 2005. - 280 p.
- Puchkov K.V., Bakov V.S., Ivanov V.V. Simultaneous laparoscopic surgical interventions in surgery and gynecology: Monograph. - M.: ID MEDPRACTIKA - M. - 2005. - 168 p.
- Puchkov K.V., Ivanov V.V., Usachev I.A. The use of polypropylene implants with simultaneous prolapse of pelvic organs // 9th Moscow. international congr. in endoscopic surgery, Moscow, April 6-8. 2005: abstract. report / ed. Yu.I. Galingera. – M., 2005. – P.296-297.
- Puchkov KV, Ivanov VV, Barsukov VA, Filimonov VB Determining of safety of soldering of vessels in tissue mass of various types while using the technique of dosed ligating electrothermal influence of ligasure // Abstracts of The 10th World Congress of Endoscopic Surgery, 13 -16 September, 2006, Berlin. – P.288.
- Puchkov K.V., Chernousova N.M., Filimonov V.B., Vasin R.V., Andreeva Yu.E. Correction of genital prolapse and stress urinary incontinence in women using modern synthetic implants // Journal. obstetrics and women's diseases.-2007.-T. 57 (special issue). — P.204-206.
- Puchkov KV, Filimonov VB, Vasin RV Correction of pelvic floors prolapse and women under tension enuresis // Abstracts book of the 16th EAES Congress 2008 with the help of up-to-date implants, 11-14 July, 2008, Stockholm, Sweden. – P.208-209.
- Puchkov K.V., Filimonov V.B., Vasin R.V., Vasina I.V. Complications of using polypropylene implants for pelvic prolapse // Journal. obstetrics and women's diseases.-2009.-T. 58.(issue 5). — P.66-67.
- Puchkov K.V., Filimonov V.B., Vasin R.V., Vasina I.V. Diagnostic capabilities and value of ultrasonography and magnetic resonance imaging for pelvic prolapse // Journal. obstetrics and women's diseases.-2009.-T. 58. (issue 5). — P.67-68.
- Puchkov KV, Filimonov VB, Vasin RV, Vasin IV Polypropylene implants as the treatment decision for patients with pelvic prolapses, connective tissue displasia and stress incontinence // Abstracts book of the 17th EAES Congress 2009, 17-20 June, 2009, Prague , Czech Republic. – P.96.
- Puchkov KV, Podzolkova NM, Andreeva JE, Dobychina AV, Korennaya VV Polypropylene implants as the treatment decision for patients with pelvic prolapses // 21st ESGE Annual Congress (Paris, 11-14 September 2012) - P.88-89.
Prevention of genital prolapse
To avoid having to resort to such radical measures as surgical removal of the uterus, you should follow a few simple rules.
- Avoid heavy physical labor and carrying heavy objects.
- During pregnancy and after the birth of the child, follow all recommendations of your doctor.
- Eat right and avoid obesity.
- Lead an active lifestyle, exercise, and swimming.
- Visit your gynecologist annually.
- At the slightest deviation and the initial stages of genital prolapse, perform special sets of exercises (Kegel gymnastics).