Obstetric hemorrhage is a leading cause of maternal mortality in many countries and a leading cause of perinatal morbidity and mortality. The etiological factors of bleeding during pregnancy depend on the gestational age.
The most common causes of bleeding in the first half of pregnancy are spontaneous abortions, ectopic pregnancy and even physiological pregnancy. Bleeding in the third trimester of pregnancy is observed in 3-4% of cases and can have obstetric and non-obstetric causes. Obstetric hemorrhages can occur antepartum and postpartum. The main causes of obstetric hemorrhage in the third trimester of pregnancy are placenta previa (20% of all cases of obstetric hemorrhage) and premature abruption of a normally located placenta (30% of cases).
- Placenta previa
- Premature placental abruption
- Hemorrhagic shock
- Uterine rupture
- Rupture of fetal vessels
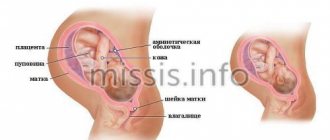
Placenta previa
Terminology . Placenta previa occurs due to abnormal implantation above the internal cervical os. There are several types of placenta previa:
Complete placenta previa - the placenta completely covers the internal os.
Partial placenta previa - the placenta partially covers the internal os.
Marginal placenta previa - the edge of the placenta reaches the edge of the internal os.
Low placental attachment (low placentation) - the placenta is located in the lower uterine segment, but does not reach the edge of the internal os.
Bleeding with placenta previa occurs due to partial detachment of small sections of placenta during normal development and thinning of the lower segment of the uterus in the third trimester of pregnancy.
Bleeding from placenta previa can become profuse and lead to hemorrhagic shock, maternal and perinatal morbidity and mortality. Perinatal mortality due to this pathology is 10 times higher than in the general population. A significant portion of the risk to the fetus associated with placenta previa is preterm delivery (60% of perinatal mortality cases), as well as other associated fetal complications.
Complications from the fetus with placenta previa
- Premature birth and its complications
- Premature rupture of membranes during premature pregnancy
- Intrauterine growth restriction
- Anomalies of fetal position and presentation
- Vaso previa of the umbilical cord
- Congenital anomalies
previa can be complicated by pathological invasion of the placenta into the uterine wall:
1) placenta accreta - pathological invasion of the placenta into the superficial layer of the myometrium with complete or partial absence of the basal decidua;
2) adult placenta - pathological invasion of the placenta throughout the entire thickness of the myometrium;
3) sprouted placenta - pathological invasion of the placenta with through penetration into the myometrium and perimeter, sometimes with penetration into nearby structures (bladder, etc.).
Placenta accreta leads to the inability of the placenta to separate from the uterine wall after birth, which can lead to massive bleeding and shock, hence maternal morbidity, disability and mortality. About two-thirds of patients with placenta previa and accompanying placenta accreta require a hysterectomy at birth (postpartum hysterectomy).
Other placental abnormalities that may cause antepartum hemorrhage include the rarer conditions:
Shaft-like placenta - the membranes double behind its edge, forming a dense ring around the periphery of the placenta. Often associated with premature placental abruption.
Blanket-shaped placenta - the fetal vessels pass between the amnion and chorion, away from the edge of the placenta, so they are exposed and more susceptible to compression and injury.
A reserve placenta is an additional portion of the placenta that is implanted at some distance from the rest of the placenta. The fetal vessels may pass between two parts of the placenta, possibly over the cervix, leaving them exposed and increasing the risk of damage.
Vaso previa of the umbilical cord is a membrane-like attachment of the umbilical cord when the fetal vessels pass over the internal os.
Epidemiology
Placenta previa occurs in 0.5% of pregnancies (1,200 births) and accounts for 20% of cases of antepartum hemorrhage. Placenta previa is associated with placenta accreta in 5-15% of cases. The risk of placenta accreta increases in patients with a previous cesarean section (25-30%), especially with a repeat previous cesarean section (50-65%).
Pathogenesis
Anomalies of placentation are the consequence of phenomena that interfere with the normal migration of the placenta during the development of the lower uterine segment as pregnancy progresses.
Factors that contribute to the development of placenta previa
- Previous caesarean section
- Previous uterine surgery (myomectomy)
- Abnormalities of the uterus
- Repeated births
- Multiple pregnancy
- Erythroblastosis
- Smoking
- History of placenta previa
- Mother's age is more than 30 years
Previous abnormal placental implantation and previous cesarean section increase the risk of abnormal placentation in subsequent pregnancies. Previous uterine surgery (myomectomy), uterine abnormalities, repeat births, older maternal age, smoking, and a history of placenta previa are also risk factors.
On the other hand, low placental attachment and even marginal presentation are not uncommon during routine ultrasound examinations in the second trimester of pregnancy. Most of these cases regress spontaneously (the phenomenon of “migration” of the placenta during the third trimester of pregnancy with the development of the lower uterine segment).
Clinic
Pregnant women with placenta previa develop sudden, profuse vaginal bleeding. The first episode of bleeding usually occurs after 28 weeks of gestation. During this period, the lower uterine segment expands and thins, disrupting the connection between the placenta and the uterine wall and causing bleeding. In some cases, patients may develop hematuria or rectal bleeding.
Objective examination
If placenta previa is suspected, vaginal examination is contraindicated due to the risk of increasing the area of placenta separation during digital examination and the development of catastrophic bleeding. Most of these patients have preliminary results of an ultrasound examination, which indicates the presence of placenta previa.
In the absence of ultrasound equipment and if placenta previa is suspected, a careful vaginal examination is performed when the operating room is ready for urgent surgery. During a vaginal examination, soft, spongy tissue may be detected near or in the area of the internal os. Due to the increase in vascularization, when examining the cervix in speculum and digital examination, pronounced varicose veins of the lower uterine segment or cervix can be seen.
Diagnostics
The diagnosis of placenta previa should be determined by ultrasound (diagnostic accuracy >95%). If the diagnosis is made before the beginning of the third trimester of pregnancy, ultrasound monitoring (a series of ultrasonographic studies) is prescribed to monitor placental migration. Transvaginal sonography should not be performed in patients with confirmed or suspected placenta previa.
If ultrasound is performed with a full bladder, placenta previa may be overdiagnosed due to compression of the lower uterine segment.
Treatment
In the third trimester of pregnancy, patients with placenta previa are prescribed strict bed rest, sexual intercourse is prohibited, and usually hospitalized after the first episode of spotting.
The development of labor, fetal hypoxia and increased bleeding are indications for urgent cesarean section, regardless of the gestational age of the fetus (according to vital indications from the mother). With mild bleeding and immaturity of the fetus, active expectant management is possible. About 70% of patients with placenta previa have recurrent episodes of bleeding and require delivery before 36 weeks of gestation. For patients in whom labor can be delayed until 36 weeks' gestation, amniocentesis is performed to determine the maturity of the fetal lungs. If sufficient lung maturity is confirmed, a cesarean section is performed between 36 and 37 weeks of gestation.
The doctor’s algorithm for placenta previa includes the following measures:
1. Stabilization of the patient’s vital functions (hospitalization, catheterization of central or peripheral veins, intravenous infusion therapy to normalize hemodynamics, fetal monitoring). Studies of blood group, Rh factor, coagulogram parameters (prothrombin time, partial thromboplastin time, fibrinogen, fibrinogen degradation products).
Rh-negative patients undergo a Kleiner-Betke test for the presence of fetal red blood cells (the degree of fetomaternal transfusion determines the number of doses of anti-Rhesus immunoglobulin required to prevent alloimmunization).
2. Preparation for massive bleeding. Expectant management, provided the patient’s condition is stable, involves hospitalization and strict bed rest. Blood substitutes (Refortan, etc.), blood (at least two bottles), plasma are prepared, and compatibility tests are carried out.
3. Preparation for premature birth. When the gestation period is less than 34 weeks, dexamethasone or betamethasone is prescribed to accelerate the maturation of the fetal lungs. To continue pregnancy, it is possible to use tocolysis with β-adrenergic agonists.
Premature placental abruption
Premature placental abruption (PAP) is the premature separation of a normally implanted placenta from the uterine wall, causing bleeding between the uterine wall and the placenta. About 50% of cases of premature abruption of a normally located placenta occur before 30 weeks of gestation, 15% during childbirth, and 30% are diagnosed only after birth when examining the surface of the placenta. Detachment of a significant surface of the placenta can lead to premature labor, uterine tetany, the development of disseminated intravascular coagulation and hypovolemic shock.
The primary cause of placental abruption is unknown, although the condition is associated with numerous risk factors and precipitating factors. These factors include maternal hypertension, previous placental abruption, maternal cocaine use, external trauma, and rapid decompression of an overdistended uterus.
At the beginning of placental abruption, blood does not clot and flows out of the placental abruption site. The growing amount of blood can cause further separation of more of the placenta. In 20% of cases of premature placental abruption, bleeding is limited to the uterine cavity (hidden, internal). In the remaining 80% of cases of placental abruption, blood leaks into the cervix and open, or external, bleeding occurs. Due to the possible leakage of blood during open bleeding, the presence of a large retroplacental hematoma, which can lead to fetal death, is less likely.
Bleeding from placental abruption leads to maternal anemia; more severe cases may be complicated by hypovolemic shock, acute renal failure and maternal death. Fetal death occurs in 35% of cases with clinically diagnosed premature placental abruption and in 50-60% of cases with severe forms. The cause of fetal death is acute hypoxia due to a decrease in the surface area of the placenta and maternal bleeding.
Epidemiology
Placental abruption occurs in 0.5-1.5% of all pregnancies and accounts for 30% of cases of bleeding in the third trimester and 15% of cases of perinatal mortality. The most common risk factors for premature placental abruption are maternal hypertensive diseases (chronic hypertension or preeclampsia).
Severe cases of placental abruption, accompanied by fetal death, are associated with maternal hypertension in 50% of cases: 25% with chronic hypertension and 25% with preeclampsia. The risk of recurrent PVP is 10%, after two cases of premature placental abruption in the anamnesis, this risk increases to 25% of cases.
Clinic
The classic symptoms of premature placental abruption are vaginal bleeding in the third trimester of pregnancy, which is accompanied by abdominal pain or tenderness of the uterus on palpation and frequent, strong contractions of the uterus. But about 30% of cases of premature placental abruption (when a small part of the placenta is detached) are asymptomatic or have mild clinical symptoms and are diagnosed only when examining the placenta after birth.
Objective examination . When examining patients with premature placental abruption, vaginal bleeding and a hard, painful uterus are usually found. Tocometry reveals both frequent short uterine contractions and tetanic contractions.
When monitoring fetal heart rate, adverse changes are observed that are a consequence of hypoxia. The classic symptom of placental abruption, which occurs during cesarean section, is the penetration (extravasation) of the myometrium with blood, which can reach the serous lining of the uterus - Couveler's uterus. The amount of blood in Kuveler's uterus does not usually interfere with postpartum uterine contractions or increase the risk of postpartum hemorrhage. In the United States, in the absence of other complications (for example, coagulation disorders), Couveler's uterus is not an indication for hysterectomy.
Diagnostics
The diagnosis of premature placental abruption is based primarily on clinical data. Only 2% of cases of PVP are diagnosed by ultrasound (visualization of retroplacental hematoma). But, given that PVP may have clinical symptoms similar to those of placenta previa, ultrasound examination is performed to exclude the diagnosis of PVP. The presence of premature placental abruption is confirmed by examining the surface of the placenta after delivery. The presence of a retroplacental clot (hematoma) with destruction of the subordinate area of the placenta confirms the diagnosis.
Treatment
Given the possibility of rapid development of catastrophic complications with PVP (massive bleeding, disseminated intravascular coagulation, fetal hypoxia), urgent delivery is usually performed by cesarean section. But in some cases, placental abruption is minor, does not lead to serious complications and does not require immediate delivery.
The doctor’s algorithm for suspected premature placental abruption includes the following points:
1. Stabilization of the patient’s vital functions. Urgent hospitalization, catheterization of central or peripheral veins, implementation of fetal heart rate monitoring. Laboratory examination: general blood test - blood group and Rh factor, coagulogram (prothrombin time, partial thromboplastin time, level of fibrinogen and fibrinogen degradation products. Rh-negative patients are prescribed anti-Rhesus immunoglobulin to prevent alloimmunization.
2. Preparing for possible massive bleeding. Implementation of standard anti-shock measures (vein catheterization, infusion of solutions, collection of blood, plasma, compatibility tests. Total blood loss with premature placental abruption is usually more expected (“hidden” bleeding).
3. Preparation for premature birth. To accelerate the maturation of the fetal lungs, dexamethasone (betamethasone) is prescribed; If the patient’s condition is stable and the fetus is immature, tocolysis can be performed to continue pregnancy up to 34 weeks.
4. Delivery in case of increased bleeding or fetal hypoxia. Urgent delivery by cesarean section is performed in patients with a threat of massive bleeding, unstable condition, or when initial signs of coagulopathy appear. With minor controlled bleeding, absence of fetal hypoxia, coagulation disorders and expectation of an early birth, vaginal delivery from a previous amniotomy is possible.
It is believed that amniotomy helps reduce extravasation of blood into the myometrium and limits the entry of thromboplastic substances into the maternal circulation. In case of fetal hypoxia, the method of choice will be cesarean section.
Diagnosis of postpartum hemorrhage
Nowadays, specialists in modern gynecology assess the risks of postpartum hemorrhage. The procedure includes direct monitoring of the level of hemoglobin in the blood, the number of red blood cells, platelets, and the state of the blood coagulation system. Atony and hypotension of the uterus should be diagnosed directly in the third stage of labor, taking into account flabbiness, the duration of the afterbirth period, as well as weak contractions of the myometrium.
Postpartum hemorrhage can be diagnosed based on thorough examinations to determine the integrity of the placenta, membranes and birth canal: whether there are any injuries, scratches or cracks. Using general anesthesia, the gynecologist can carefully perform a manual examination, examining the uterine cavity and determining whether there are ruptures, parts of the placenta, blood, defects, soil for the further development of tumors, diseases, as well as reasons that prevent the contraction of the myometrium.
One cannot fail to mention the importance of the role of timely prevention of late postpartum hemorrhage through ultrasound of the pelvic organs, which must be performed 2-3 days after birth. It is this procedure that allows you to detect fragments of unnecessary tissue in the body directly in the woman’s uterus.
Hemorrhagic shock
A severe maternal complication of massive bleeding is hemorrhagic (hypovolemic) shock. With blood loss of more than 25% of the circulating blood volume (CBV), or more than 1500 ml, the clinical picture of hemorrhagic shock develops. In 10% of cases of PVP that resulted in fetal death, that is, with abruption of > 2/3 of the placenta, the development of disseminated intravascular coagulation, or disseminated intravascular coagulation syndrome (DIC) is possible.
This syndrome develops as a result of the entry of massive doses of tissue thromboplastin (from places of damage to the placenta) into the maternal vascular system, which promotes the activation of the coagulation cascade, primarily in the microvascular bed. This leads to the development of ischemic necrosis of parenchymal organs - kidneys, liver, adrenal glands, pituitary gland.
Ischemic renal necrosis can develop as a result of acute tubular necrosis or bilateral cortical necrosis and manifests with oliguria and anuria. Bilateral cortical necrosis is a fatal complication that requires hemodialysis and can lead to death of the woman due to uremia in 1-2 weeks.
Management of patients with hypovolemic shock requires rapid restoration of lost blood volume. Central venous catheterization is performed, central venous pressure is measured to monitor the restoration of blood loss, a catheter is inserted into the bladder to monitor diuresis, oxygen inhalation is introduced, and infusion of blood and blood substitutes is started until the NET level is more than 30% and urine output is > 0.5 ml/kg / hour Studies of platelet counts, fibrinogen levels and serum potassium are performed after infusion of every 4-6 vials of blood.
Blood coagulation tests (tests for DIC) are carried out every 4 hours before delivery. The most sensitive clinical test for the development of DIC syndrome is the level of fibrinogen degradation products (FDP), although only a single study of the FDP level has prognostic significance, i.e. Based on the level of PDF, one cannot draw a conclusion about the effectiveness of treatment. Although normal PDP results do not exclude the possibility of DIC, fibrinogen levels and platelet counts are the most important markers of DIC.
Urgent delivery is the main component of the treatment of DIC syndrome, and leads to regression of its manifestations. The method of choice is caesarean section. If the fetus dies and the patient's condition is stable, vaginal delivery is possible. If the platelet count is <50,000 or the fibrinogen level is <1 g/L, these blood components must be restored. Restoration of fibrinogen levels is achieved by transfusion of fresh frozen plasma or cryoprecipitate. Heparin is not usually used.
Uterine rupture
Pathogenesis . Uterine rupture is an obstetric disaster that often leads to maternal and fetal death. Most uterine ruptures occur during childbirth. More than 90% of uterine ruptures are associated with a previous cesarean section or other operations on the uterus (conservative myomectomy, etc.). In the remaining 10% of cases, uterine rupture occurs in the absence of a history of any scarring on the uterus.
In these cases, uterine rupture may be forced, associated with abdominal trauma (car accident, external and internal obstetric rotation of the fetus) or labor and delivery (high doses of oxytocin, excess pressure on the fundus of the uterus), or spontaneous, “histopathic” (with placenta accreta, multiple pregnancy, in multiparous older women, with hydatidiform mole, choriocarcinoma).
When the uterus is completely ruptured, all layers of the uterine wall are damaged, including the serosa; if incomplete, the integrity of the serous layer is not compromised.
The main complications for the mother with uterine rupture are massive bleeding and hypovolemic shock. Uterine rupture among the causes of maternal mortality is 1%, but it increases significantly with delayed obstetric care. Perinatal mortality with this complication can exceed 15% of cases.
Epidemiology . Uterine rupture is a rare complication and accounts for 1:15,000 of all births in patients who have not had previous uterine surgery. Risk factors for this complication are conditions that are accompanied by damage or thinning of the walls of the uterus (scars on the uterus, overdistension of the uterus, inadequate use of high doses of uterotonics during childbirth, congenital anomalies of the uterus and placentation abnormalities).
factors for uterine rupture
- Previous uterine surgery
- Use of inappropriate doses of oxytocin
- Multiparous older women
- Significant stretching of the uterine walls
- Fetal position anomalies
- Large fruit
- External or internal obstetric rotation of the fetus
- Injury
Clinic . Clinical symptoms of uterine rupture are highly variable. Classic symptoms are sudden acute abdominal pain, vaginal bleeding (from slight to massive), changes in the contours of the uterus and removal of the presenting part of the fetus, cessation of labor, acute hypoxia, or fetal death.
Treatment for uterine rupture involves emergency delivery by laparotomy. If possible, the site of uterine rupture is sutured and complete hemostasis is achieved; in other cases, a hysterectomy is performed. If the uterus is preserved and pregnancy follows, delivery of such patients is performed by cesarean section at 36 weeks of gestation when the maturity of the fetal lungs is confirmed.
Preventing postpartum bleeding problems
If a woman in labor has an obstetric and gynecological history, has disturbances in the functioning of the coagulation system, is taking anticoagulants, or has a high risk of developing postpartum hemorrhage, then she must be under medical supervision both during pregnancy and after it. With such disorders, women in labor are sent to special maternity hospitals.
To prevent postpartum hemorrhage, women are given medications that promote normal uterine contractions. In the first few hours after birth, women in labor need to be under the close supervision of doctors so that they can accurately assess the amount of uterine bleeding after childbirth at its early stage.