Piracezin - a drug to improve blood circulation and memory
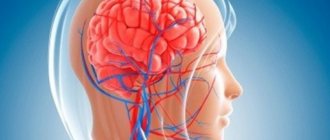
Piracezin is a combined (piracetam + cinnarizine) nootropic drug with a pronounced antihypoxic and vasodilating effect, improving cerebral circulation and cerebral metabolism.
Piracetam belongs to the class of nootropic substances that have a positive effect on the metabolic processes of the brain. It increases the concentration of ATP in brain tissue, enhances the biosynthesis of ribonucleic acid and phospholipids, stimulates glycolytic processes, and enhances glucose utilization. Piracetam improves the integrative activity of the brain, promotes memory consolidation, has a protective effect in various forms of cerebral hypoxia, and facilitates the learning process.
Piracetam is well absorbed when taken orally. When introduced into the body, it penetrates into various organs and tissues, including brain tissue. Practically not metabolized. Excreted through the kidneys. It is slightly toxic (in acute experiments on animals, the lethal dose exceeds 10 g/kg when administered intravenously).
Piracetam is indicated for the treatment of cortical myoclonus as mono- or complex therapy, complex therapy of sickle cell anemia.
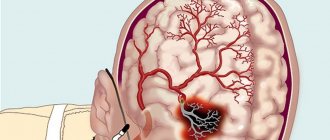
In neurological practice, piracetam is used to treat neurological, intellectual-mnestic and emotional-volitional disorders in patients with cerebral infarction, traumatic brain injury, dyscirculatory and post-traumatic encephalopathy, and consequences of stroke.
In pediatric practice, piracetam is used when necessary to accelerate the learning process and eliminate the consequences of perinatal brain damage caused by intrauterine infection, hypoxia, birth trauma, mental retardation, mental retardation, and cerebral palsy.
In psychiatric practice, piracetam is used for neurotic and asthenic depressive states of various origins with a predominance in the clinical picture of signs of adynamia, asthenic syndrome, organic syndromes with impaired memory and attention.
In chronic alcoholism, piracetam is prescribed to reduce the phenomena of asthenia, intellectual-mnestic and other mental disorders.
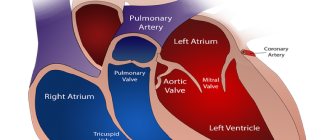
Cinnarizine improves cerebral, peripheral and coronary circulation and microcirculation, increases the ability of red blood cells to deform, reduces high blood viscosity and increases tissue resistance to hypoxia.
It also has a direct antispasmodic effect on blood vessels, reduces their response to biogenic vasoconstrictors (adrenaline, norepinephrine), and potentiates the effect of CO2 on brain vessels. Cinnarizine does not significantly affect systemic blood pressure, heart rate, contractility and cardiac conductivity.
Cinnarizine has moderate antihistamine activity, reduces the excitability of the vestibular apparatus (suppresses nystagmus).
The pharmacological properties of cinnarizine are largely due to its ability to block membrane calcium channels. It inhibits the entry of calcium ions into cells and reduces their content in the plasma membrane depot.
Cinnarizine is well absorbed. When taken orally, peak plasma concentrations are observed after 1-3 hours; half-life is about 4 hours. It is actively metabolized. A third of the metabolites are excreted in the urine, and 2/3 in the feces.
Cinnarizine is used for disorders of cerebral and peripheral circulation, vestibular disorders. Despite the presence of antihistamine activity, it is not used specifically as an antihistamine due to the availability of more effective drugs.
As a cerebrovascular agent, it is prescribed for cerebral circulatory disorders associated with vasospasm, atherosclerosis, traumatic brain injury, and stroke. The drug reduces cerebroasthenic phenomena, headaches, tinnitus, and improves general condition.
Cinnarizine is prescribed for migraines and Meniere's disease.
Cinnarizine also reduces spasms of peripheral vessels and improves blood circulation in obliterating endarteritis, thromboangiitis, Raynaud's disease, intermittent claudication, acrocyanosis and other disorders of peripheral circulation.
When piracetam and cinnarizine are combined, both components mutually potentiate each other's action without increasing toxicity, since the toxicity of the combination does not exceed the toxicity of the individual components.
With regard to the effect of the drug on the central nervous system, the sedative effect of cinnarizine predominates. The therapeutic effect of the drug appears after 1-6 hours.
Piracezin capsules 400mg/25mg No. 10x6
Name
Pyracesin
Description
Hard gelatin capsules of cylindrical shape with hemispherical ends, white.
Main active ingredient
Piracetam+cinnarizine
Release form
Hard gelatin capsules, 10 capsules in a blister pack, 1 or 6 blister packs per pack.
Dosage
400 mg+25 mg
special instructions
Since Piracezin® contains cinnarizine, the drug can cause a positive reaction in athletes during a doping test. Piracesin® is the drug of choice in cases where monotherapy with piracetam causes tension and insomnia in the patient. When treating patients with cortical myoclonus, abrupt cessation of treatment should be avoided (risk of resumption of attacks). It should be borne in mind that the development of side effects is most typical for patients with mental disorders. Exacerbation of coronary insufficiency occurs more often in severely ill patients; in these cases, the dose should be reduced or therapy discontinued. Due to the antihistamine activity of cinnarizine, the drug may interfere with the results of allergy skin tests performed within 4 days after taking Piracezin®. Due to the effect of piracetam on platelet aggregation, caution should be exercised when prescribing the drug to patients with hemostasis disorders, bleeding symptoms, before upcoming surgery, previous surgery (including dental), risk factors for bleeding (for example, gastric and duodenal ulcers). intestines), previous intracerebral hemorrhage, taking anticoagulants or antiplatelet agents, including low doses of aspirin. In patients with Parkinson's disease, cinnarizine should only be prescribed if the therapeutic benefits outweigh the possible risk of exacerbation of the disease. Like other antihistamines, cinnarizine can cause irritation in the epigastrium; taking the drug after meals can reduce the effects of gastric irritation. Cinnarizine may cause drowsiness, especially early in treatment. Therefore, concomitant use of alcohol or CNS depressants should be avoided. Cinnarizine should be avoided for porphyria.
Indications for use
Indications for the use of the drug are: symptomatic treatment of memory disorders, intellectual impairments in the absence of a diagnosis of dementia (symptomatic treatment of chronic psychoorganic syndrome); symptomatic treatment of labyrinthine and vestibular disorders of vascular origin, Meniere's syndrome.
Directions for use and doses
For adults, the drug is prescribed 1-2 capsules 3 times a day for 1-3 months, depending on the severity of the disease. The duration of the course of treatment is determined by the doctor, taking into account the characteristics of the disease, the achieved effect and tolerability of the drug. Since piracetam is excreted from the body by the kidneys, when prescribing the drug to patients with renal failure and elderly patients, the dose should be adjusted depending on the value of creatinine clearance (CC). Patients with renal failure require dose adjustment of the drug in accordance with the following scheme: Degree of renal failure QC (ml/min) Dose Normal > 80 Usual dose Mild 50–79 2/3 of the usual dose in 2–3 doses Moderate 30–49 1/ 3 usual doses in 2 divided doses Severe
Use during pregnancy and lactation
The drug is contraindicated for use in pregnant women and breastfeeding women.
Precautionary measures
Use in children The use of the drug in children under 18 years of age is not recommended due to the lack of adequate data. Impact on the ability to drive a car, work with equipment While taking the medicine, it is recommended to refrain from driving a vehicle and working with potentially dangerous mechanisms.
Interaction with other drugs
The possibility of changing the pharmacokinetics of piracetam under the influence of other drugs is low, since 90% of piracetam is excreted unchanged in the urine. When used concomitantly with thyroid hormones, there have been reports of confusion, irritability, and sleep disturbances. According to a study of patients with recurrent venous thrombosis, piracetam at a dose of 9.6 g/day increases the effectiveness of indirect anticoagulants (there was a more pronounced decrease in platelet aggregation, fibrinogen concentration, von Willebrand factors, blood and plasma viscosity compared with the use of acenocoumarol alone). At concentrations of 142, 426 and 1422 μg/ml, piracetam does not inhibit cytochrome P450 isoenzymes. At a concentration of 1422 µg/ml, minimal inhibition of CYP 2A6 (21%) and 3A4/5 (11%) was observed. However, normal inhibition constant values can probably be achieved at higher concentrations. Thus, metabolic interaction of piracetam with other drugs is unlikely. Taking piracetam at a dose of 20 g/day for 4 weeks did not change the maximum serum concentration and the area under the concentration-time curve of antiepileptic drugs (carbamazepine, phenytoin, phenobarbital, valproate) in patients with epilepsy receiving piracetam at a stable dose. Concomitant use of alcohol, CNS depressants, tricyclic antidepressants may enhance the sedative effects of these drugs or cinnarizine. Due to the antihistamine effect, cinnarizine may mask a positive reaction during a skin test, so its use should be discontinued 4 days before the test.
Contraindications
Individual intolerance to piracetam or pyrrolidone derivatives, as well as other components of the drug; psychomotor agitation at the time of drug prescription; Huntington's chorea; acute cerebrovascular accident (hemorrhagic stroke); parkinsonism; end-stage chronic renal failure (with creatinine clearance less than 20 ml/min); pregnancy, breastfeeding period.
Compound
One capsule contains: piracetam – 400 mg, cinnarizine – 25 mg. Excipients: calcium stearate, magnesium carbonate basic. Capsule shell composition: gelatin, glycerin, purified water, titanium dioxide, sodium lauryl sulfate.
Overdose
Symptoms: changes in consciousness from drowsiness to stupor and coma, vomiting, extrapyramidal symptoms, arterial hypotension, convulsions, dyspeptic symptoms in the form of diarrhea with blood and pain in the abdomen. Treatment: there is no specific antidote. During the first hour after ingestion, gastric lavage is necessary. If warranted, activated charcoal may be prescribed. Treatment is symptomatic.
Side effect
From the blood and lymphatic system: bleeding disorders From the immune system: anaphylaxis, hypersensitivity reactions Mental disorders: restlessness, depression, agitation, anxiety, confusion, hallucinations From the nervous system: hyperactivity, drowsiness, ataxia, balance disorders, convulsions , exacerbation of epilepsy, headaches, insomnia, tremor, dyskinesia, extrapyramidal disorders, parkinsonism From the organ of hearing and balance: dizziness Gastrointestinal disorders: feeling of dry mouth, abdominal pain, pain in the upper abdomen, diarrhea, nausea, vomiting, cholestatic jaundice. Diseases of the skin and subcutaneous tissue: Quincke's edema, dermatitis, itching, urticaria, hyperhidrosis, lichen keratosis, lichen planus, lupus-like reaction Disorders of the reproductive system and mammary glands: increased libido General disorders: weight gain, asthenia, muscle rigidity. If any adverse reactions occur, including those not listed in this leaflet, you should stop taking the medicine and consult a doctor.
Storage conditions
Store in a place protected from moisture and light at a temperature of 15 to 25 °C. Store out of the reach of children. Shelf life: 2 years. Do not use after the expiration date stated on the packaging.
Buy Piracezin caps.400mg/25mg in container pack No. 10x6 in the pharmacy
Price for Piracezin caps. 400 mg/25 mg in container pack No. 10x6
Instructions for use for Piracezin caps. 400 mg/25 mg in container pack No. 10x6