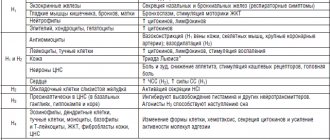
Chemical properties
Calcium Carbonate, what is it? This is an inorganic chemical. compound, salt formed by Ca and carbonic acid . Chemical formula of Calcium Carbonate: CaCO3. In nature, the substance can be found in calcite, vaterite, aragonite, marble, limestone, ordinary chalk, and eggshells. This is a fairly common mineral; according to its chemical formula, it has three polymorphic modifications.
In appearance - odorless white crystals or fine powder. The substance is insoluble in water, alcohol, and soluble in dilute nitric and hydrochloric acid (this releases carbon dioxide). According to Wikipedia, molar mass of the substance = 100.1 grams per mole.
Calcium carbonate is made from minerals, mainly marble. In laboratory conditions, the compound can be obtained using the calcination reaction of Ca oxide, resulting in the formation of Ca hydroxide , through which CO2 is passed and carbonate . The chemical properties are characterized by the decomposition reaction of Calcium Carbonate, in which, under the influence of high temperature, the substance is split into quicklime and carbon dioxide. Also, the chemical compound is characterized by a reaction with water and CO2, with the formation of Ca bicarbonate .
The product is used:
- for the production of paint, plastics, household chemicals and rubber;
- as food coloring E170 ;
- in the production of chalk for writing on a blackboard, plastics;
- in whitewashing for trees, ceilings and gardening;
- in the food and paper industries;
- in paper production (bleach, deoxidizer, filler);
- as a source of calcium in the production of tableware, fiberglass, glass products;
- in medicine.
A differentiated approach to the selection of second-generation soluble calcium preparations
Insufficient supply of the body with calcium ions is a risk factor for a number of chronic pathologies, including disorders of bone metabolism, muscle functioning, endothelium, immune and cardiovascular systems. According to the World Health Organization, calcium content in drinking water is one of the fundamental factors determining human health at the population level [1].
However, drinking water in the vast majority of geographic regions contains an amount of calcium that is obviously insufficient to compensate for the body's daily needs (1000 mg/day for adults). In addition, drinking water available to most people undergoes multiple purification cycles to remove organic impurities and toxic “heavy” metals - lead, mercury, cadmium. During cleaning, the already low calcium content is significantly reduced. For this reason, there is a need to saturate drinking water with macro- and microelements necessary for health, primarily calcium, using special preparations.
From a pharmacological point of view, the intake of calcium into the body in the form of an aqueous solution has a number of significant advantages. First, the calcium is already in a dissolved state (unlike, say, insoluble calcium carbonate tablets). Secondly, in the aqueous solution there are no ligands that interfere with the absorption of calcium (for example, food products contain a significant amount of phytic acid, which converts calcium ions into an insoluble and poorly digestible form). Thirdly, the supply of water with calcium ions in the drug solution helps solve the problem of insufficient fluid intake (an adult should drink at least 2–2.5 l/day of clean drinking water). Fourthly, an aqueous solution of calcium can be enriched with special synergistic micronutrients that improve the pharmacokinetics and pharmacodynamics of calcium.
This paper discusses the features of calcium preparations that must be taken into account when prescribing them so that their use is as effective and safe as possible. The results of studies of the calcium supply of various populations, the pharmacology of various calcium preparations and the intended purpose of soluble organic calcium preparations are consistently reviewed. Data from clinical and epidemiological studies and evidence-based medicine are presented.
Calcium supply in different populations
The recommended daily intake (RDI) of calcium in the Russian Federation is on average 1000 mg/day for adults, for people over 60 years old - 1200 mg/day. The physiological need for children is from 400 to 1200 mg/day [2]. RSPs in other countries lie in comparable ranges of values (Table, Fig. 1).
Clinical and epidemiological studies conducted in different countries show that the average daily calcium intake among various segments of the population is 500–1000 mg/day and rarely exceeds the RDI.
Thus, available clinical and epidemiological data indicate that calcium intake is insufficient. Normalization of calcium intake can be carried out both by changing the composition of the consumed diet and by using special calcium supplements.
Clinical pharmacology of calcium preparations based on inorganic and organic salts
Compensation for calcium deficiency can be carried out both by changing the composition of the consumed diet and by using special calcium supplements.
A significant problem for carrying out the most effective and safe compensation for dietary calcium deficiency is the choice of pharmacological substance (calcium salt) and pharmaceutical form of calcium (tablets, dragees, drinking solution). This section provides prolegomena for a differentiated approach to the selection of safe and effective calcium preparations.
The absorption of calcium from drugs depends on factors such as 1) the calcium substance, 2) the dose of calcium, 3) the mode of administration, 4) the pH of the gastric juice and, of course, 5) combined use with other drugs. According to the substance, there are inorganic (1st generation) and organic (2nd generation) calcium preparations. The vast majority of 1st generation calcium preparations are calcium carbonate, which is insoluble in water and requires a certain pH range for absorption. Organic calcium preparations (2nd generation) are better soluble in water, significantly less dependent on the pH of gastric juice, and are characterized by higher bioavailability than tablet forms [4].
Inorganic calcium salts
Calcium carbonate
Calcium carbonate is the most common one of the cheapest forms of calcium, which is widely used in medicine as an antacid [5]. Antacids act on the surface of the gastric mucosa, neutralizing hydrochloric acid in gastric juice (HCl). When hydrochloric acid is neutralized, the calcium carbonate portion of the antacid dissolves according to the equation:
CaCO3 + 2HCl → CaCl2 + CO2↑+H2O
The resulting calcium chloride is absorbed by the epithelial cells of the stomach and enters the blood. Most of the calcium carbonate remains undissolved and moves through intestinal transit into the large intestine. Therefore, the absorption of calcium carbonate largely depends on the acidity of the stomach (better absorption is observed at lower pH [6]) - after all, calcium carbonate is insoluble in water and its absorption in the body occurs exclusively through interaction with HCl of gastric juice. Significant absorption of calcium from calcium carbonate should only be expected in patients with hyperacidity of the stomach.
Organic calcium salts
Calcium lactate
Calcium lactate is a characteristic component of mature cheeses. Calcium lactate can be absorbed at different pH levels, and this form of calcium can be taken without regard to meals. Calcium lactate successfully neutralizes the insufficient effects of estrogen-containing drugs on bone mineral density [7]. However, taking calcium lactate preparations alone cannot ensure complete satisfaction of calcium needs.
Calcium gluconate
Calcium gluconate is used as a topical agent or in the form of an injection solution [8, 9]. With a certain method of treatment, calcium gluconate is able to physiologically stimulate the release of the active form of the calcitonin molecule from parafollicular C-cells [10], and is characterized by a clear positive effect on renal function, exhibiting vasodilatory and natriuretic properties [11]. Calcium gluconate is also an effective and safe oral form of calcium for increasing bone mass density in preterm infants [12].
Calcium citrate
Calcium citrate is an exceptionally effective and safe form of calcium. First, calcium citrate is completely soluble in water. Secondly, calcium from citrate is absorbed regardless of food intake or the acidity of gastric juice. The chemical properties of calcium citrate make it the drug of first choice in patients with low gastric acidity, as well as in elderly patients and patients using antacids and proton pump inhibitors. Thirdly, the citrate anion itself has its own physiological significance, being the main substrate of the central energy cycle of each cell - the Krebs cycle. Fourth, the citrate anion helps reduce the risk of kidney stones and, moreover, promotes the dissolution of kidney stones [13].
The results of a comparison of the solubility of several calcium preparations of the 1st and 2nd generations in a wide pH range (from 1 to 7) showed that tablets of 1st generation preparations based on calcium carbonate disintegrate within 7–9 minutes and do not completely dissolve even at low pH values (i.e. high acidity) [14]. On the contrary, the 2nd generation preparation studied (calcium carbonate mixed with citric acid and calcium lactogluconate) completely dissolves within 2–3 minutes, forming a clear solution of calcium citrate that does not contain sediment. The time for complete dissolution of the 2nd generation drug also increased slightly with increasing pH (i.e., a decrease in acidity), although not as pronounced (pH = 1.39 - 2 min 10 s, pH = 7.04 - 3 min, i.e. e. less than 1 minute, p < 0.002 (Fig. 2).
As noted above, an important factor in the absorption of calcium carbonate-based drugs is the acidity of gastric juice. The composition of gastric juice includes hydrochloric acid (providing a low pH value), gastric juice enzymes pepsin, gastrixin, mucus, minerals (potassium, ammonium and sodium chlorides, sulfates, phosphates), etc. A significant difference between 1st generation drugs based on calcium carbonate is that they almost completely (up to pH values = 7) neutralize hydrochloric acid when simulating the pH of gastric juice. At the same time, a preparation based on calcium citrate forms a slightly acidic solution with a very stable pH value = 4.52 ± 0.15 at any initial pH (i.e., it can be absorbed both at high, normal and low acidity of the stomach). The 2nd generation drug based on calcium citrate formed a slightly acidic solution with pH values lying in a very narrow range - pH = 4.52 ± 0.15 (Fig. 3) [14].
Calculations have shown that even at pH < 2.0, no more than 50% of calcium carbonate from tablets of 1st generation drugs passes into the solution, and the solubility of the tablet drops sharply with increasing pH (i.e. with a decrease in the acidity of gastric juice). From the 2nd generation drug, all 100% of calcium goes into solution, which contributes to a significant increase in the absorption of calcium in the body (Fig. 4) [14].
On the intended purpose of soluble organic calcium preparations
Available basic research and evidence-based medicine have shown a number of distinct benefits from the use of calcium lactate, gluconate and calcium citrate. The introduction of drugs based on the above organic salts corresponds to the basic principle of clinical pharmacology - maximum efficiency and maximum safety. This principle corresponds to the soluble calcium preparation based on organic salts Calcium Sandoz® Forte and dietary supplements (BAA) Calcium D3 Sandoz® Osteo and Calcium Sandoz® Beauty. They are available in the form of tablets (500 or 1000 mg of elemental calcium and 600 mg for Calcium D3 Sandoz® Osteo) for the preparation of an aqueous solution, which the patient receives. Taking these medications will also, in part, help compensate for insufficient drinking water consumption (Fig. 5).
Calcium Sandoz® Forte contains three calcium salts (calcium lactate, calcium gluconate and calcium carbonate). 1 tablet with 500 mg of calcium contains an equimolar mixture of calcium lactate and calcium gluconate in the amount of 1132 mg, calcium carbonate 875 mg (total 500 mg of elemental calcium) and 1662 mg of citric acid. It should be emphasized that the tablets of the drug are used to prepare a drinking solution, which the patient consumes. During the preparation of an aqueous solution, a chemical interaction of calcium carbonate with citric acid occurs with the formation of calcium citrate and the volatilization of the resulting carbon dioxide from the solution:
3CaCO3 + 2C6H8O7 → Ca3(C6H5O7)2 + 3CO2↑+ 3H2O
When a tablet of the drug is dissolved in water, the resulting solution contains calcium cations surrounded by anions of organic acids (lactate, gluconate and citrate), which stabilize calcium ions in the solution and ensure good bioavailability of the ionized form of calcium. For this reason, Calcium Sandoz® Forte is the drug of choice for the prevention of calcium deficiency.
Calcium D3 Sandoz® Osteo contains 600 mg/tablet. elemental calcium in the form of citrate, lactate and gluconate, 400 IU/table. vitamin D and is intended to reduce the risk of osteoporosis, including increasing bone mineral density and reducing the risk of fractures associated with calcium deficiency; to maintain normal levels of calcium and vitamin D in order to reduce the risk of developing osteoporosis in the pre- and postmenopausal period. As is known, risk factors for the development of osteoporosis and osteoporotic fractures are gender (female), age (over 65 years), body mass index less than or equal to 20 kg/m2, estrogen deficiency, sedentary lifestyle and diet deficient in calcium and vitamin D [15 ].
The synergy of the properties of the components of Calcium D3 Sandoz® Osteo, which offers both the consumption of highly digestible organic calcium salts and vitamin D, allows it to be taken to compensate for calcium deficiency and conditions associated with a deficiency of this element: to increase bone mineral density and reduce the risk of fractures, for reducing the risk of developing osteoporosis in the pre- and postmenopausal period.
Calcium citrate (1200 mg/day) in combination with vitamin D (800 mg/day) reduces the risk of fractures [16]. The meta-analysis included 17 placebo-controlled studies (n = 52,625) and showed that the combination of calcium salts with vitamin D reduced the risk of all types of fractures by 13% [17]. A comparative meta-analysis of 15 clinical studies that compared the bioavailability of calcium carbonate and calcium citrate included a total of 184 patients and showed better bioavailability of calcium citrate. According to the results of the meta-analysis, calcium absorption from calcium citrate was significantly higher than from calcium carbonate: by 20% on average in the sample, by 24% in category “A”, by 27% when taken on an empty stomach and by 22% when taken on an empty stomach. taken with meals [18].
Citrate anion itself helps normalize bone metabolism. A randomized study of elderly patients without osteoporosis (n = 201, 65 years and older) showed that long-term intake of potassium citrate (4.5 g/day for 2 years) increased bone mineral density (average 1.7 ± 1. 5%, 95% confidence interval (CI) 1.0–2.3, p < 0.001), and this effect apparently relates specifically to the effect of the citrate anion [19].
Among other things, calcium citrate in combination with vitamin D has a beneficial effect on the safety of teeth. In a study of a group of 145 older adults aged 65 years and older, patients were randomized to receive either calcium citrate 500 mg/day or 1000 mg/day (calculated as elemental calcium) and vitamin D (700 IU/day) within 2-year osteoporosis prevention program. Among patients receiving calcium 500 mg/day, 40 of 68 participants (59%) experienced at least one tooth loss over the 2-year follow-up period. At the same time, only 33 of 77 patients (40%) receiving calcium at a dose of 1000 mg/day. tooth loss was noted. Thus, taking calcium citrate in combination with vitamin D reduced the risk of tooth loss by 50% (OR 0.5, 95% CI 0.2–0.9) [20].
Conclusion
Correcting calcium deficiency is of vital importance to maintaining health. Replenishing dietary calcium deficiency to prevent metabolic disorders of bone tissue, skin, and hair can be done using various calcium salts. The significant difference between inorganic salts (calcium carbonate, calcium phosphate) and organic calcium salts (citrate, lactate, calcium gluconate) is their solubility and therefore the bioavailability of calcium.
Due to its low cost and relative effectiveness, calcium carbonate antacid has long been used not only for its intended purpose, but also to compensate for calcium deficiency. At the same time, the absorption of calcium from carbonate significantly depends on the state of the gastrointestinal tract and, above all, on the acidity of the stomach (since calcium carbonate is practically insoluble in water).
Calcium carbonate is contraindicated in patients with low stomach acidity, with polyps of the stomach and intestines. Taking calcium carbonate is obviously undesirable while taking estrogen-containing drugs. Data from pharmacology, experimental and clinical medicine indicate the effectiveness and safety of using organic calcium salts such as lactate, gluconate and citrate to correct calcium deficiency (Fig. 6). Among organic salts, calcium citrate is characterized by the highest solubility (the solubility line is as follows: calcium citrate > calcium lactate > calcium gluconate).
Soluble calcium preparations based on organic salts, produced, in particular, in the form of effervescent tablets, are completely soluble in water and therefore are much better absorbed than insoluble calcium carbonate or phosphate. Calcium Sandoz® Forte (1000 mg/table. elemental calcium in the form of organic salts) can be used for the prevention and treatment of calcium deficiency in a wide range of patients, including pregnant women, women during lactation and children after three years of age. Dietary supplement Calcium D3 Sandoz® Osteo (organic calcium salts in combination with vitamin D) is intended to reduce the risk of osteoporosis, including increasing bone mineral density and reducing the risk of fractures associated with calcium deficiency; to maintain normal levels of calcium and vitamin D in order to reduce the risk of developing osteoporosis in the pre- and postmenopausal period. Thus, in modern pharmacology there has been a transition to the most natural compensation of calcium deficiency in the form of the use of aqueous solutions of completely soluble and quickly digestible calcium citrate.
Literature
- World Health Organization. Calcium and Magnesiumindrinkingwater, 2009, p. 194.
- Norms of physiological needs for energy and nutrients for various groups of the population of the Russian Federation, MR 2.3.1.2432–08.
- Maltsev S.V., Arkhipova N.N., Shakirova E.M. Vitamin D, calcium and phosphates in healthy children and in pathology. Kazan, 2012, 45 p.
- Gromova O. A., Volkov A. Yu., Torshin I. Yu., Gromov A. N., Nosikov V. V., Gogoleva I. V. Comparative analysis of the solubility of various calcium preparations depending on the acidity of the medium // Doctor. 2013, 7, p. 18–24.
- Richard W. Lime Kilns and Lime Burning. 2004. P. 4. ISBN 978–0-7478–0596–0.
- Lieberman H. A., Leon Lachman, Joseph B. Schwartz (1990). Pharmaceutical Dosage Forms: Tablets. New York: Dekker. P. 153. ISBN 0–8247–8044–2
- Mizunuma H., Okano H., Soda M., Tokizawa S., Kagami I., Miyamoto S., Honjo S., Ibuki Y. Calcium supplements increase bone mineral density in women with low serum calcium levels during long-term estrogen therapy // Endocr J. 1996; 43 (4): 411–415.
- Yoshimura CA, Mathieu L., Hall AH, Monteiro MG, de Almeida DM Seventy per cent hydrofluoric acid burns: delayed decontamination with hexafluorine ® and treatment with calcium gluconate // J Burn Care Res. 2011; 32(4):e149–54 doi.
- Capitani EM, Hirano ES, ZuimIde S., Bertanha L., Vieira RJ, Madureira PR, Bucaretchi F. Fingerburnscausedbyconcentratedhydrofluoricacid, treatedwithintra-arterialcalciumgluconateinfusion: casereport // Sao Paulo Med J. 2009; 127(6):379–381.
- Giovanella L. Serum procalcitonin and calcitonin normal values before and after calcium gluconate infusion // Exp Clin Endocrinol Diabetes. 2012; 120 (3): 169–70 doi.
- Ruilope LM, Oliet A., Alcazar JM, Hernandez E., Andres A., Rodicio JL, Garcia-Robles R., Martinez J., Lahera V., Romero JC Characterization of the renal effects of an intravenous calcium gluconate infusion in normotensive volunteers // J Hypertens Suppl. 1989; 7(6):S170-S171.
- Horsman A., Ryan SW, Congdon PJ, Truscott JG, Simpson M. Bone mineral accretion rate and calcium intake in preterm infants // Arch Dis Child. 1989; 64 (7) Spec: 910–918.
- Torshin I. Yu., Gromova O. A. 25 moments of molecular pharmacology. A-griff, 2012. 695 p.
- Gromova O., Volkov A., Torshin I., Gromov A., Nosikov V., Gogoleva I. Comparative analysis of the solubility of various calcium preparations depending on the acidity of the medium // Doctor. 2013. No. 7. pp. 18–24.
- Toroptsova N.V., Benevolenskaya L.I. Osteoporosis: modern approaches in the prevention of osteoporosis and fractures // Breast Cancer. 2003, No. 7, p. 398
- Quesada Gomez JM, Blanch Rubio J., Diaz Curiel M., Diez Perez A. Calcium citrate and vitamin D in the treatment of osteoporosis // Clin Drug Investig. 2011; 31 (5): 285–98 doi.
- Tang BM, Eslick GD, Nowson C., Smith C., Bensoussan A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis // Lancet. 2007, Aug 25; 370(9588):657–666.
- Sakhaee K., Bhuket T., Adams-Huet B., Rao DS Meta-analysis of calcium bioavailability: a comparison of calcium citrate with calcium carbonate // Am J Ther. 1999; 6 (6): 313–321.
- Jehle S., Hulter HN, Krapf R. Effect of potassium citrate on bone density, microarchitecture, and fracture risk in healthy older adults without osteoporosis: a randomized controlled trial // J ClinEndocrinolMetab. 2013; 98(1):207–217.
- Krall EA, Wehler C., Garcia RI, Harris SS, Dawson-Hughes B. Calcium and vitamin D supplements reduce tooth loss in the elderly // Am J Med. 2001; 111(6):452–456.
- Limanova O. A., Torshin I. Yu., Sardaryan I. S., Kalacheva A. G., Hababpashev A., Karpuchin D., Kudrin A., Yudina N. V., Egorova E. Yu., Grishina T R., Gromov A.N., Fedotova L.E., Rudakov K.V., Gromova O.A. Micronutrient provision and women’s health: establishing relationships based on intellectual analysis of clinical and epidemiological data // Issues of gynecology, obstetrics and perinatology. 2014, vol. 13, no. 2, p. 5–15.
O. A. Gromova*, 1, Doctor of Medical Sciences, Professor I. Yu. Torshin**, Candidate of Chemical Sciences A. V. Pronin* E. Yu. Egorova*, Candidate of Medical Sciences A. Yu. Volkov***
* State Budgetary Educational Institution of Higher Professional Education IvSMA Ministry of Health of the Russian Federation, Ivanovo ** RSC International Institute of Trace Elements UNESCO, Moscow *** State Budgetary Educational Institution of Higher Professional Education Russian National Research Medical University named after. N. I. Pirogova Ministry of Health of the Russian Federation, Moscow
1 Contact information
Indications for use
Calcium carbonate is prescribed:
- for diseases of the digestive tract with hyperacidity of gastric juice ( gastritis , gastric ulcer , acute duodenitis , symptomatic ulcer of any origin, gastrointestinal erosion heartburn , reflux esophagitis );
- patients with osteoporosis , including during postmenopause ;
- for caries and rickets in childhood;
- patients with osteomalacia and tetany ;
- pregnant women , during breastfeeding;
- during the period of intensive growth of the child;
- with hypocalcemia after long-term treatment with GCs, with renal osteodystrophy , hypoparathyroidism , slow calcium absorption;
- as part of a comprehensive allergy .
Who are calcium supplements indicated for?
- In the treatment of pathological fractures due to osteoporosis
- Women over 50 years of age with vitamin D deficiency or deficiency
- If you have osteoporosis
- For women who have reached menopause to prevent osteoporosis
- If you have insufficient calcium intake from food:
The daily requirement for calcium in children under 3 years of age is 700 mg, for children 4-10 years of age and adults 1000 mg. An increased requirement of up to 1200 mg is observed in women in menopause or over 50 years of age and in men over 70 years of age. Adolescents during the growing period (10-16 years), pregnant and breastfeeding women should receive up to 1300 mg of calcium.
Overdose
hyperkacemia may occur (taking more than 2 grams per day). Symptoms: general weakness, headaches , anorexia , lack of appetite, vomiting, constipation , thirst, lethargy, polyuria , pain in the joints and muscles, heart rhythm disturbances, kidney disease.
It is recommended to rinse the stomach, give the victim enterosorbents , and carry out symptomatic treatment.
Use of E170 additive as an ingredient in food products
The food industry practically cannot do without white chalk, because it is completely harmless to humans and is approved for use by children. As a leavening agent, coloring agent, stabilizer, it is used to prepare:
- baby food;
- concentrated cream and milk;
- chocolate;
- hard cheeses.
The additive is also used to process grape juice.
Interaction
Combination use of the drug with tetracycline antibiotics can lead to a decrease in their effectiveness and plasma concentration.
When combining the drug with thiazide diuretics, there is a higher risk of developing metabolic alkalosis and hypercalcemia .
Calcium carbonate slows down the absorption processes of other drugs.
of indomethacin increases .
When combined with levothyroxine , it reduces the effect of taking an anabolic steroid.
Cosmetics industry - in which products is the substance found?
White chalk is one of the components of creams, powders and foundations for the face, mattifying lotions, body creams and scrubs, blush, concealers, hair dyes, and children's cosmetics. The substance has the property of absorbing excess sebum, thereby removing oily shine and evening out skin tone. As a thickener and stabilizer, calcium carbonate improves the consistency of cosmetic products, promotes the absorption of excess water and prevents the appearance of lumps. As a weak abrasive component, it cleans and adds shine, which is why it is used in the production of toothpastes. Naturally, the coloring effect of white chalk is also used by manufacturers of cosmetics - with its help they give products a white color.
Drugs containing (Analogs)
Trade names of the product: Calcium carbonate , Vitacalcin , Additiva calcium , Scoralite , UPSAVIT Calcium . In combination with magnesium carbonate : Tums , Rennie , Rumney , Andrews antacid . In combination with colecalciferol, the drug is included in the following medications: Ideos , Revital Calcium D3 , Calcium-D3 Nycomed , Natekal D3 , Complivit calcium D3 . With magnesium hydroxide : Gastrik , Vitrum Mag . Complex products: Alfadol-Sa , Vitrum Beauty Lux , Vitrum Osteomag , Gaviscon , Kalcemin and so on.
What tests to take
It is difficult to detect calcium deficiency using a blood test, because the body does everything possible to maintain a constant level of ionized calcium in the blood. But it’s easy to be deficient or deficient in vitamin D by taking a blood test for 25-OH vitamin D.
To identify contraindications and evaluate the effectiveness of treatment with calcium preparations, you should take tests:
- Calcium and phosphorus in the blood
- Calcium in daily urine
- Blood creatinine
- 25-OH vitamin D
In the Lab4U laboratory this can be done with a discount of up to 50%.
Construction and chemical industries and calcium carbonate
The substance is used as a component of putties and various sealants in the production of glass, paper, household chemicals, paints (technical and artistic), and plastics. For the production of the latter, about 45-50% of all white chalk in the world is mined. As a filler and dye, it is used to produce polyvinyl chloride, polyolefins, and polyester fibers.
For agriculture, calcium carbonate is a means to cleanse the soil and restore its acid-base balance.
Similar drugs:
- Maalox Oral suspension
- Almagel Neo Oral suspension
- Maalox Chewable tablets
- Phosphalugel Gel for oral administration
- Gaviscon Oral suspension
- Almagel A Oral suspension
- Gastrofarm Oral tablets
- Gaviscon Forte Oral suspension
- Sodium bicarbonate Solution for infusion
- Sodium hydrocarbonate Solution for infusion
** The Drug Directory is intended for informational purposes only. For more complete information, please refer to the manufacturer's instructions. Do not self-medicate; Before starting to use the drug Calcium carbonate, you should consult a doctor. EUROLAB is not responsible for the consequences caused by the use of information posted on the portal. Any information on the site does not replace medical advice and cannot serve as a guarantee of the positive effect of the drug.
Are you interested in the drug Calcium carbonate? Do you want to know more detailed information or do you need a doctor's examination? Or do you need an inspection? You can make an appointment with a doctor - the Euro lab is always at your service! The best doctors will examine you, advise you, provide the necessary assistance and make a diagnosis. You can also call a doctor at home . Euro lab clinic is open for you around the clock.
** Attention! The information presented in this medication guide is intended for medical professionals and should not be used as a basis for self-medication. The description of the drug Calcium carbonate is provided for informational purposes and is not intended for prescribing treatment without the participation of a doctor. Patients need to consult a specialist!
If you are interested in any other drugs and medications, their descriptions and instructions for use, information about the composition and form of release, indications for use and side effects, methods of use, prices and reviews of drugs, or you have any other questions and suggestions - write to us, we will definitely try to help you.