Release form and composition
Dosage form - emulsion for intravenous administration: after thawing - transparent, with a bluish tint, odorless (50, 100, 200 or 400 ml in glass bottles, sealed with rubber caps, crimped aluminum caps).
100 ml of emulsion contains:
- Pfocalin – 13 g;
- Pforidin – 6.5 g;
- Proxanol – 4 g;
- Sodium chloride – 0.6 g;
- Glucose – 0.2 g;
- Sodium bicarbonate – 0.065 g;
- Potassium chloride – 0.039 g;
- Magnesium chloride – 0.019 g (in terms of dry matter);
- Sodium phosphate monosubstituted – 0.02 g.
Excipient: water for injection.
Indications for use
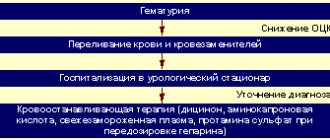
- Acute and chronic hypovolemia (due to traumatic brain injury, hemorrhagic, infectious-toxic, burn or traumatic shock, as well as surgical and postoperative hypovolemia);
- Impaired microcirculation and peripheral circulation (with infections, fat embolism, cerebrovascular accident, purulent-septic conditions, changes in gas exchange and tissue metabolism);
- Regional perfusion;
- Lung lavage;
- Purulent wounds of the abdominal and other cavities;
- Anti-ischemic protection of donor organs (preliminary preparation of the recipient and donor).
Directions for use and dosage
Perftoran is intended for intravenous (IV) administration.
Before using the drug, the doctor conducts a biological test: slowly injects 5 drops of the drug, takes a 3-minute break, then injects another 30 drops and again takes a 3-minute break. If there are no adverse reactions, administration is continued.
Before infusion, the doctor conducts a visual inspection of the drug. It is considered suitable for use if there are no cracks on the bottle, the closure is sealed and the label is intact.
The doctor records the data from the Perftoran label (name, manufacturer, batch number) and visual inspection of the bottles, as well as the results of the biological test in the medical history.
Recommended application regimens:
- Acute blood loss and shock: intravenous drip or jet at a dose of 5-30 mg/kg. The maximum effect can be achieved if the patient breathes an oxygen-enriched mixture during the infusion and for 24 hours after it;
- Microcirculation disorders: a single dose is 5-8 ml/kg. The drug can be re-administered at the same dose 3 times at 2-4 day intervals. To improve the oxygenation effect during treatment, it is recommended to supply an oxygen-enriched air mixture through a nasal catheter or mask;
- Anti-ischemic protection of donor organs: intravenous drip or jet to the recipient and donor at a dose of 20 ml/kg 2 hours before surgery;
- Regional use: for perfusion of extremities when filling a standard oxygenator at a dose of 40 ml/kg;
- Topical application: similar to traditional drug therapy.
Clinical trials of perftoran (PF) in Russia began in 1984. Phase 3 clinical trials and additional trials for individual indications were completed. Perftoran has been registered and officially used in the clinic since 1996. PF or the so-called “blue blood” - a blood and plasma substitute with a gas transport function - has a multifunctional effect.
The main aspects of the action are as follows:
1.Improving the delivery of oxygen from the alveoli to erythrocytes and from erythrocytes to tissues. 2.Improving metabolism and gas exchange at the tissue level. 3. Restoration of central hemodynamics. 4.Improving blood flow up to peripheral microcirculation. 5. Distinct cardioprotective effect. 6. Activation of the detoxifying function of the liver. 7. Membrane stabilizing effect. 8.Has sorption and diuretic properties. 9. Immunomodulatory effect
Pharmacokinetic features of the drug:
All components of PF are chemically inert and do not undergo metabolic transformations in the body. The half-life of perfluorocarbon emulsions from the bloodstream is 20-30 hours. The half-life of a surfactant from the bloodstream is approximately 6 hours. The surfactant is excreted through the kidneys. More than 90% of perfluorocarbons are excreted through the lungs, a small part through the skin, from the liver with bile, and about 1% through exocytosis. Temporarily, 20-30% of perfluorocarbons are accumulated by macrophages.
Indications for use of PF
As a blood substitute with the function of transporting oxygen and carbon dioxide to reduce the cost of donor blood and red blood cells as an anti-shock, anti-ischemic and cardioprotective agent
1. in urgent situations: - with traumatic, hemorrhagic, burn, cardiogenic, infectious-toxic shock; - in comatose states of various origins (be it cerebral coma due to TBI, stroke; hepatic coma as a result of viral or toxic hepatitis, cirrhosis; renal coma as a result of acute or chronic renal failure); - with blood loss, both acute (wound, traumatic, operating) and chronic, accompanied by severe hypovolemia; - in purulent-septic conditions and severe infectious diseases with the simultaneous use of powerful antibiotics, drainage and sanitation of foci of infection; - with the threat of development and already developed embolism (thromboembolism, fat embolism), disseminated intravascular coagulation syndrome, respiratory distress syndrome; - for burn disease; 2. For combined polytraumas leading to the development of one or another urgent situation. 3. For open heart surgery using a heart-lung machine (CAB), for coronary artery bypass grafting, for cardioplegia during reconstructive operations on the heart and blood vessels. 4. In transplantology to protect donor organs before, during and after transplantation. 5. In the clinic of internal diseases - in cardiac patients (in the acute stage of myocardial infarction); - for vascular and inflammatory diseases of the brain; - for vascular diseases of the extremities; - for diseases of the gastrointestinal tract (erosive gastroduodenitis, peptic ulcer of the stomach and duodenum); 6. In case of severe complicated course of various infectious diseases (viral hepatitis, abdominal, typhus, relapsing fever, fever, meningo-encephalitis), with AIDS. 7. In psychiatry for the relief of delirium tremens and acute psychoses. 8. For oncological diseases, both during surgical treatment and to reduce the adverse effects of radiation and chemotherapy. 9. In toxicology in case of poisoning with neurotropic and hepatotropic poisons to reduce the phenomena of endotoxicosis. 10. For local use: - regional perfusion; - lung lavage; — washing of trophic, purulent and burn wounds; — irrigation of cervical erosions; - per os and through a tube for erosive gastritis and massive gastric ulcers and 12pcs; — washing of the pleural and abdominal cavities during surgical treatment. 11. Liquid perfluorocarbons have found wide application in eye microsurgery.
Page 30
Doses and methods of administration of perftoran
PF is administered intravenously by stream and drip at a rate of 1 to 30 ml per 1 kg of weight (and more in case of acute massive blood loss). It is possible to re-administer the drug in the same dose at intervals from several hours (in case of massive blood loss) to 3 days, depending on the patient’s condition, the results of the administration of previous doses and the type of pathology, unless this is a surgical intervention accompanied by premedication and anesthesia. Before administering the main amount of the drug (if there is no premedication or anesthesia), a biological test is necessary: after administering the first 5 - 30 drops of PF, you need to take a break for 15 - 20 minutes to identify possible anaphylactoid reactions. In the absence of the latter, you can continue the infusion at an arbitrary pace, on average at a rate of 40-60 drops per 1 minute.
Undesirable side effects of PF
When administering a test dose, some people may experience headache, dizziness, chest pain, difficulty breathing, cough, pain in the lumbar region, itching, chills; hyperemia of the face and skin of the body, increased temperature, increased heart rate, decreased blood pressure. If these phenomena do not go away spontaneously within 10-15 minutes, then it is better to discontinue the drug or administer PF under the cover of antiallergic drugs. In case of a weakly expressed vascular vasomotor reaction that spontaneously passes within 10 - 15 minutes in the form of slight redness of the skin, mild rash, scratching of the nose, dizziness, slight tachycardia up to 90 beats per minute, increased intestinal motility, the administration of the drug should be continued, but in a slow manner pace (20-30 drops per minute). possible and necessary The frequency of adverse reactions when administering PF depends on the type of pathology. No one has registered any adverse reactions in polytrauma, blood loss compensation, cardiodioplegia, or in transplantology. In “therapeutic” situations, the percentage of adverse reactions ranges from 1.8% to 15% and depends on the type of pathology, preliminary selection of patients, compliance with the rules and storage periods, defrosting technology and the number of repeated freezing of the drug, and on compliance with precautions when administering PF.
Contraindications
Contraindications for the administration of the drug are: individual intolerance, hemophilia (since there are no comprehensive studies on the effect of PF on hemostasis), the first two thirds of pregnancy (in the absence of vital indications). We also consider a relative contraindication if the patient has a history of autoimmune diseases and severe hysteria.
Precautionary measures when administering perftoran.
PF cannot be administered in the same system, or in the same syringe, or in the same AIK with dextrans (polyglucin, rheopolyglucin), hydroxyethyl starch with a molecular weight of more than 100,000 Da. These drugs should be administered after the end of the PF infusion or, if simultaneous administration is necessary, into another vein. PF is compatible with donor blood, components of donor blood, albumin, isotonic saline solutions, glucose, AB, antispasmodics, analgesics, cardiotonic drugs, known blood substitutes such as mafusol, polyoxidine.
Release and storage form
PF is available in the form of an odorless, white emulsion with a bluish tint, in glass bottles of 100, 200 and 400 ml. It is planned to produce smaller volume bottles for use in pediatrics and geriatrics. The drug must be stored frozen at temperatures from -4 to -18oC. The shelf life when frozen is 3 years; when thawed at a temperature of +4 oC, it is advisable to store the drug for no more than two weeks. It is important to follow the technology for defrosting the drug; in particular, you should not sharply change the temperature regime: 12 hours before the intended use, the bottle with PF is transferred from the freezer to the refrigerator at a temperature of +4 oC, then the drug is “warmed up” immediately before use to the temperature of the intended administration in warm water or an oxygenator (+ 14 oC is required for hypothermic cardioplegia, +24-26 oC for general hypothermia, +33-36 oC for normothermic use). It is not recommended to shake the bottle sharply, which must be taken into account both during transportation and when defrosting the drug. Thawed PF is stored at a temperature of +4oC if it is intended to be used within the next 2 weeks. We believe that refreezing the drug should not be more than 3 times.
Advantages of PF over donor blood, plasma and blood components
1.PF is suitable for patients with any blood group and any Rh factor. 2.The possibility of transferring infectious and viral diseases is excluded. 3. The consumption of donor blood in case of massive blood loss is significantly reduced. 4. Long-term storage of the drug and its factory production in the required quantities are possible, regardless of the availability of biological donors. 5. Delivery of oxygen to places inaccessible to red blood cells through spasmodic and narrowed vessels. By the way, it is precisely this property of PF that allows one to avoid the development or reduce the manifestation of respiratory distress syndrome.
Page 34
Some specific examples of the effectiveness of PF when used in the clinic.
Compensation for acute massive blood loss (25% or more of the total blood volume). PF is administered drip and stream, intravenously and even intravenously. If the patient’s condition allows, a biological test is carried out; if the patient’s condition is critical, premedication is given (glucocorticosteroids, antihistamines, Ca drugs, sedatives), and cardiotonic drugs are also available. The volume of injected PF is 10-15% greater than the volume of blood loss if only PF is replaced. If there is simultaneous replacement of blood loss with polymer plasma substitutes (dextran, albumin, hydroethyl starch), then they are administered through another system into the other arm or after completion of the administration of PF. In this case, the amount of administered PF will be 50-60% of the volume of blood loss, and the volume of polymer plasma substitutes will also be 50-60%. With massive blood loss of more than 30% of the total blood volume, it becomes necessary to also use whole blood. Compensation for blood loss begins with the introduction of PF in a volume of 40-50% of the volume of blood loss, then or in parallel, a plasma substitute is injected into the other arm in the same volume, and then donor blood or red blood cells are injected; in this case, the required amount of donor blood is reduced by 2-3 times. If bleeding continues, platelet mass is also administered. PF can be re-introduced depending on the patient’s condition and replenishment of blood loss. In case of polytrauma with massive blood loss, we have experience in administering up to 5.4 liters of PF to the patient. There are many examples of the introduction of PF on the battlefield in an amount of 1.2 liters with blood loss up to 50% of the blood volume - these are cases during the war in Afghanistan in 1985-86. In this situation, in addition to 1.2 l of PF, 1.2 l of blood and up to 1 l of polyglucin were administered. In this case, the oxygen content in the inhaled air mixture (Fi O2 indicator) is made equal to 0.4 - 0.6 (40 - 60 vol.% oxygen). I would like to remind you that after the introduction of PF, the alveolar-arterial O2 gradient decreases, which is very important for preventing the development and reducing the manifestation of respiratory distress syndrome.; — central hemodynamics improves (min. volume increases due to increased stroke volume of the heart and improved functioning of the left ventricle; blood pressure increases; pressure in the pulmonary artery decreases; venous pressure decreases); - improves cerebral and renal blood flow; — peripheral blood flow and tissue microcirculation improves; — acidosis is reduced (by improving the delivery of oxygen to tissues and the intake of carbon dioxide from them); — oxygen delivery by erythrocytes improves, which leads to a decrease in peripheral vascular resistance and normalization of the arteriovenous difference in pO2 and pCO2.
Use of PF in the treatment of obliterating vascular lesions of the extremities.
Due to the ability of PF to improve microcirculation and rheological properties of blood, relieve spasms, improve oxygen delivery by red blood cells and pass through narrowed vessels where red blood cells themselves cannot pass, PF has found application in vascular lesions of the extremities.
With this pathology, the first priority comes to the problem of selecting patients based on medical history (absence of allergic and autoimmune diseases) and the mandatory conduct of a biological test. The presence of minor vasomotor reactions listed above, which disappear after 10-15 minutes, is not a contraindication for the administration of PF. To date, more than 350 patients with obliterating atherosclerosis have been treated, 180 of whom have been treated for over 4 years. Order of introduction.
On the first day - 300 - 400 ml IV, drip. During administration, the patient can be given to breathe an air-oxygen mixture with an O2 content of 40 - 60%. Repeated administration of the same dose is indicated after 1 - 3 days, 1 - 2 times. The results of treatment are assessed by changes in well-being and by objective data, in particular by increasing the distance that the patient can walk without pain; according to a transcutaneous sensor that records pO2 on the skin and in the underlying tissues, according to thermal imaging and Doppler measurements. PF courses can be repeated every 2-3 months. To date, four years of experience in treating patients with stage 11 obliterating atherosclerosis have been accumulated. according to Fontaine. In several patients with 111 st. The disease was successfully canceled or surgical treatment was postponed. The percentage of severe anaphylactoid reactions, due to which the use of PF had to be discontinued, in this group of patients was equal to two.
Page 35
Application of PF in cardiac surgery.
The greatest experience in the use of PF in cardiac surgery has now been accumulated at the Institute of Surgery named after. Vishnevsky, Institute of Cardiovascular Surgery named after. Bakulev, at the Transplantation Center with V.I. Shumakov and at the Military Medical Academy of St. Petersburg. PF has been used in reconstructive surgeries on the heart and blood vessels using AICA for perfusion preservation of the heart, for cardioplegia only PF and PF together with conventional crystalloid compounds. When only PF is used for cardioplegia, its salt composition is adjusted by adding salts and substrates to a cardioplegic composition. If PF is used as a component, then it is diluted with a cardioplegic composition in a ratio of 1:1 to 1:3. The positive effect is more pronounced when using a solid PF and consists of a 2-3 fold increase in the time between perfusions; — when the heart is connected to the bloodstream, spontaneous (independent) restoration of electrical and contractile activity is observed (a defibrillator had to be used in isolated cases); — arrhythmia rarely developed, and the level of contractile activity was always higher when using PF; - due to the selective blocking of the incoming calcium current, the level of contracture was lower, accordingly the diastolic pressure in the aorta was lower due to a more complete diastole, and the systolic pressure was higher; — secondary development of heart failure when using Pf was usually minimal.
PF in the treatment of acute myocardial infarction.
(Used by the Dagestan Medical Academy, Makhachkala and Dnepropetrovsk Medical Academy, Ukraine, Military Medical Academy in St. Petersburg). PF was used in patients with myocardial infarction in the acute stage. The first infusion of the drug was given 30-60 minutes after the development of a heart attack, the second infusion was given the next day, and, if necessary, on the 3rd day. The amount of drug administered was determined at the rate of 1.5-2 ml per kg of patient weight. PF was administered intravenously, by drip. The total amount for a single administration did not exceed 100 ml. Before the administration of PF, patients were given premedication (50 mg of pipolfen and 30-60 mg of prednisolone). After infusion of 5-10 drops of PF, a break was taken for 3-5 minutes to identify a hidden allergic reaction; in the absence of the latter, the drug was administered at a rate of 40-60 drops per minute. With more forced administration, patients may experience tachypnea (shortness of breath), tachycardia (palpitations), and decreased blood pressure. Allergy sufferers have bronchospasm. The effectiveness of PF was assessed by the well-being and clinical condition of the patient; parameters of central hemodynamics, ECG, respiratory function, capnography, biochemical and coagulation parameters of blood were studied. The studies were carried out immediately after administration and on the third day. The examination results showed that after administration of the drug, the pain syndrome decreases, the patient’s well-being improves, on the second day there is a positive ECG dynamics, and blood rheology improves (fibrinogen levels decrease, PTI decreases). Due to the decrease in preload, the central venous pressure decreases; due to the decrease in afterload, the resistance to blood ejection from the ventricles decreases, and the peripheral vascular resistance decreases. If the beginning of PF infusion is usually accompanied by some increase in heart rate (110-120 beats per 1 min.) and respiration (19-25 per 1 min.), then subsequently these indicators level out to normal numbers. Blood pressure fluctuates slightly. The SV of the heart and SI (stroke index), MOS (cardiac minute volume) and CI (cardiac index) increase. The total and specific peripheral resistance decreases, and the central venous pressure decreases. All this undoubtedly indicates an improvement in heart function and has a beneficial effect on the course and outcome of the disease. The recovery period of patients with AMI who received PF in the acute period is reduced. Comparison of ECG and ultrasound data in patients receiving PF and patients in the control group reveals a decrease in the necrosis zone by 2.5 times.
A separate message can be devoted to the use of PF in transplantology.
PF was used mainly in kidney and testicular transplantation to improve both the quality of the graft and its safety. An agonizing donor, in whom brain death was recorded “perfectly”, was connected to a heart-lung machine and injected with up to 2 liters of PF, then polyglucin was added and the donor’s body was perfused for approximately two hours, and only then the necessary organs were removed. The organs taken in this way were distinguished by their high preservation and good survival rate, even in the case when the time of organ collection was two days behind the time of transplantation. Once connected to the recipient's bloodstream, the kidney immediately produced urine. The indicators of residual nitrogen, creatinine, and urea were restored 2-3 times faster than when using other sampling methods. When a kidney is transplanted, practically no PF enters the recipient's body, but if about 400 ml of PF is administered to the recipient after transplantation, this improves the condition of both the patient and the transplant, even if the kidney is prepared in the usual way. I would like to draw your attention to the fact that if, during perfusion of the PF of the body of the intended donor, the kidney does not produce urine after 1.5-2 hours, while still in the donor’s body, there is no point in transplanting it. This sign is used as a prognostic test. An example of the unsuccessful use of kidneys is two attempts at kidney transplantation from patients over 70 years of age who died from hemorrhagic stroke. We can talk about many other, less numerous observations of the general and local use of PF. Currently, the Pharmacocommittee has already approved the use of PF as a means of activating the detoxifying function of the liver. We will try to cover the use of PF in other clinical situations in subsequent reports.
Used Books.
1. Physiological activity of fluorine-containing compounds (experiment and clinic). Pushchino Scientific Center. Institute of Theoretical and Experimental Biophysics RAS. Pharmaceutical company ..Pushchino, 1995, 246 p. 2.Perfluoroorganic compounds in biology and medicine. Pushchino, 1997, 250 p. 3.Perfluoroorganic compounds in biology and medicine. Pushchino, 1999, 286 p. 4.Perftoran in intensive care of critical conditions. Guidelines. Dnepropetrovsk, 2000, 40 p. 5.Physiologically active substances based on perfluorocarbons in military medicine. All-Army Scientific Conference October 8-9, 1997. St. Petersburg, 1997, 152 p. 6.Physiologically active substances based on perfluorocarbons in experimental and clinical medicine. All-Army Scientific Conference September 23-24, 1999. St. Petersburg, 1999, 121 p.
Page 36
Side effects
Perftoran is generally well tolerated. In some cases, the following side effects occur:
- Allergic (redness of the skin, itching and urticaria) and anaphylactoid reactions;
- Decreased blood pressure, increased heart rate, chest pain, difficulty breathing;
- Headache, increased body temperature;
- Pain in the lumbar region.
If the described reactions occur or if complications occur, it is necessary to immediately stop the infusion and, without removing the needle from the vein, depending on the clinical picture, administer desensitizing, vasopressor, glucocorticosteroid, cardiotonic and/or other drugs used in the treatment of anaphylactic shock.
Blood substitutes - blue blood, perftoran
No, we are not talking about counts, dukes, and other nobles whose noble family dates back hundreds of years. In this case, blue blood means Perftoran. The history of the creation of this drug is at the same time interesting, controversial, tragic, and in many ways unclear to this day.
Problems of blood substitutes
Almost everyone has heard about blood substitutes, even those who have never been seriously ill or injured, and whose activities are not related to medicine. In general, these are medications in the form of solutions for infusions, intravenous drips. Saline, solutions of glucose, colloidal compounds, and other substances are used as blood substitutes.
But few people know that blood substitutes do not exist, this very concept is erroneous, and those drugs that are commonly called blood substitutes are not such.
Alas, there are still no products that can completely replace blood. Otherwise, hospitals would not need whole blood, red blood cells, or fresh frozen plasma. And all the problems associated with donation would be a thing of the past.
The infusion-administered agent fills the vascular bed, improves blood circulation or perfusion of organs and tissues, helps remove toxic substances from the body, and performs some other functions of blood, as if replacing it. That's where the name came from.
But none of the drugs is able to take on one of the main functions of the blood - transport.
Transport of oxygen to organs and tissues, and transport of carbon dioxide in the opposite direction is carried out by red blood cells. These red blood cells make up the bulk of dry blood. Having a rich yellow color, in large quantities they form the red color of blood.
This is due to the iron in hemoglobin. And if our hemoglobin contained copper instead of iron, our blood would be blue.
The number of other formed elements, leukocytes and platelets, is small in comparison with erythrocytes. Along with the formed elements, there is also a liquid part of the blood, plasma. It also dissolves oxygen. But the amount of oxygen dissolved in plasma is so insignificant that it should not be considered as an oxygen carrier.
Bleeding from fractures, wounds, and other injuries is accompanied by loss of both plasma and red blood cells. Loss of plasma has more serious consequences than loss of red blood cells.
The phenomena of hemorrhagic shock, i.e., shock due to bleeding, are observed already with blood loss of 15% of the circulating blood volume (CBV).
And if you lose 35-40% of your bcc, irreversible changes that are incompatible with life develop in organs and tissues. We cannot live with empty vessels. Therefore, at the first, pre-hospital, stage, the main task of medical care is to fill the vascular bed as quickly as possible.
For this purpose, infusions of solutions are carried out, which are designed to replenish the lack of plasma. Therefore, these solutions should not be called blood substitutes, but plasma substitutes.
What about red blood cells?
Their immediate loss in large numbers also does not bode well. There are few red blood cells - there is nothing to deliver oxygen and remove carbon dioxide from tissues. Oxygen deficiency (hypoxia) aggravates the severity of hemorrhagic shock.
To eliminate tissue hypoxia and everything associated with it, blood transfusions are performed - transfusions of whole donor blood and red blood cells.
Carrying out transfusions is associated with a number of problems:
- Transfusions should not be performed prehospital by emergency medical personnel. This activity can only be done in a hospital setting.
- The hospital does not always have a sufficient amount of donor blood, the required type and Rh factor. And sometimes it is absent altogether. This is more typical for peripheral hospitals.
- Before transfusion, it is necessary to determine the blood type, Rh factor, group, Rh, and biological compatibility. These activities require additional effort and time of medical staff, which are so necessary at a critical moment.
- There may be errors when performing compatibility tests. Transfusion of incompatible blood is fraught with post-transfusion shock with an extremely serious condition.
- Another danger is transfusion of blood infected with HIV, hepatitis, and syphilis. Of course, all donated blood is checked. But there is always a small chance of a seronegative period or an immunological window when the donor was recently infected and early tests give a negative answer.
- Some patients, for religious reasons, refuse blood transfusions even at the cost of their own lives or the lives of their loved ones.
The scale of these problems increases with mass casualties during natural disasters, military operations, and major industrial and transport accidents.
Creation of Perftoran
Perftoran was conceived in order to avoid the inconvenience and dangers associated with blood transfusions. The drug in the form of an emulsion was positioned as a true blood substitute capable of delivering oxygen to tissues.
Perftoran is based on synthetic substances, perfluorocarbons or perfluoroorganic (PFO) compounds. Natural organic compounds are made up of carbon and hydrogen. In perfluorocarbons, hydrogen atoms are replaced by fluorine atoms.
With this structure of molecules, the solubility of oxygen and carbon dioxide in a liquid PPO medium increases to such an extent that this liquid transports gases no worse, but better, than red blood cells.
The background to the creation of Perftoran began in the USA in the 60s of the last century, when a mouse was placed in a vessel with a perfluorocarbon emulsion. The animal died, but not immediately, as one would expect, but breathed in this liquid for some time.
American scientists went further and transfused PFO compounds into rats, almost completely replacing their blood. After this, the experimental animals continued to live for some time.
Scientists have assessed the prospects associated with the practical use of PPO compounds. Drugs created on their basis could be used as blood substitutes in emergency care, without resorting to blood transfusions.
Practical work has begun on the creation of drugs based on PPO compounds. The USA and Japan took the leading positions in this direction. Of the several drugs created by scientists in these countries, Fluozol-DA and Oxygen were approved for practical use.
For the first time in the history of medicine, Fluozol was used as a blood substitute in the USA to treat patients who, for religious reasons, refused donated blood.
In the USSR, PFD compounds were developed simultaneously in two scientific institutions. These are the Institute of Hematology and Blood Transfusion in Moscow, and the Institute of Biophysics of the USSR Academy of Sciences in Pushchino, near Moscow. Moreover, Pushchino employees came closest to the intended goal when they developed Perftoran.
The scientific work was headed by Professor F.F. Beloyartsev, an anesthesiologist by training, is a brilliant scientist who defended his doctoral dissertation at the age of 34. The Institute of Biophysics owes its success to him.
Work on the creation of Perftoran, which began at the very beginning of the 80s, was carried out intensively and was crowned with success. The desired emulsion was obtained. Every newly created drug is tested in laboratories on animals.
Then the Pharmaceutical Committee gives permission for clinical trials on groups of volunteers. And only after a new product has proven its effectiveness and safety is it allowed to be used in practice.
Perftoran has not undergone clinical trials and has not been officially approved. But an unexpected opportunity came up. A 5-year-old girl is hit by a moving vehicle and is taken to the hospital with serious injuries.
Here she is mistakenly transfused with incompatible blood. Post-transfusion reactions begin. A gesture of despair: the management of the clinics turns to Beloyartsev for help. And he comes to the rescue, delivers Perftoran, and the girl remains to live.
In the 80s there is a war in Afghanistan. There are many wounded, and there is not enough donor blood. And then military doctors take Perftoran to Afghan hospitals.
The drug is administered to several hundred fighters. Around the same time, he was dubbed blue blood. It is noteworthy that Perftoran emulsion is white and resembles milk in appearance. But after its injection, the veins become blue.
In 1984, permission was received from the Pharmaceutical Committee to conduct clinical trials. Beloyartsev and the group of scientists he led were nominated for the USSR State Prize. The glory of the discoverers, a revolution in medicine, universal recognition in the USSR and abroad - all this was just around the corner.
Denouement
And suddenly, like a bolt from the blue, Perftoran testing was banned. Soon, employees of the prosecutor's office, OBKHSS, and the KGB became interested in Beloyartsev's group.
Beloyartsev was accused of stealing alcohol from a laboratory, stealing drugs, and embezzling employee money. The scientist was also reminded of the practical use of a drug not approved by the Pharmaceutical Committee.
It turns out that several hundred military personnel were destined for the role of guinea pigs. Humiliating checks and searches followed one after another. Unable to withstand the persecution, Beloyartsev committed suicide. In December 1985, he was found hanged at his own dacha in Pushchino.
Of course, Beloyartsev did not steal any alcohol or drugs. There were deductions from employee bonuses for scientific development. But the scientist did not appropriate anything to himself personally. Who or what is behind the false accusations? There are many options. Perhaps this is the envy of competitors from the Institute of Hematology, or the personal resentment of one of the employees.
Conflicts between Beloyartsev and employees of state security agencies also took place. It is generally accepted that the great scientist and his brainchild joined the list of victims of the inert totalitarian Soviet system. But the true reason for the tragic outcome remained a mystery.
Alternative opinions
Perftoran itself is shrouded in a mysterious aura. Scientists have mixed views on its effectiveness and safety. Many argue, and not without reason, that the drug does more harm than good.
The predecessor of Perftoran, the above-mentioned Japanese-American Fluozol, was banned in the USA in 1994. The frequency of allergic reactions is too high, up to 35%, with a low ability to carry oxygen for a short time. Proponents of Perftoran appeal to the fact that all the disadvantages of Fluozol are due to the large size of the molecules.
At the same time, the particle size of Perftoran is only 0.1 microns. Compared to red blood cells with a size of 7 microns, they are 70 times smaller!
In shock conditions, the so-called capillary spasm develops. sludge phenomenon. Round red blood cells stick together into microscopic conglomerates, shaped like coin columns.
These columns clog the capillaries, blood does not circulate through them, and hypoxia worsens. At the same time, small particles of Perftoran penetrate through lubricated capillaries and carry oxygen even to hard-to-reach areas of tissue.
But delivering oxygen is not everything. It needs to be transferred to tissue cells. Red blood cells do this very well. But the PFD connections, according to some scientists, have problems here. They do not transfer oxygen to tissues well. If this is true, then it turns out that an oxygen-rich substance circulates through the vessels, but it is of little use.
Perftoran was positioned as an inert substance. Allegedly, it does not react with organic compounds in the blood and other tissues, and is therefore harmless. But perfluorocarbons are inherently foreign, and therefore potentially dangerous, because may negatively affect metabolism. PPO compounds are slowly eliminated from the body, accumulating in the liver, lung tissue, and bone marrow.
During the research, the dog was infused with Perftoran. The drug replaced up to 70% of the blood volume, and, despite this, the animal remained alive. True, after a while the dog died anyway. When it was opened, the liver was affected by cirrhosis. According to some data, PPO compounds are a breeding ground for bacteria and destroy surfactant. This fluid is contained in the alveoli, prevents them from sticking together, and thereby maintains gas exchange in the lungs.
Experts say that perfluorocarbons are mutagens. They damage DNA and provoke cancer. Due to teratogenicity and the risk of congenital deformities, these compounds are not recommended for women of reproductive age. Teratogenicity is another reason why Fluozol was banned.
After the use of Perftoran in Afghanistan and in a number of medical institutions in the USSR, a high mortality rate was allegedly recorded. So, out of 700 soldiers, 200 died, many of the rest developed cancer. Perhaps this is all speculation.
Then the truth could be established by objective information from documents indicating when, to whom, for what purpose Perftoran was used, and the cause of death. But this data did not exist, or for some reason it was not published.
The organofluorine compounds in Perftoran are structurally similar to polytetrafluoroethylene, better known as Teflon. It turns out that patients are injected into a vein... material for covering frying pans.
When employees of the Institute of Biophysics, inspired by the success of creating Perftoran, asked Beloyartsev to administer a miracle drug to them, the scientist replied: “What are you guys talking about, is it really possible to administer such a demo to yourself.”
And if this is not a slander, and these words were actually spoken, then the events appear in a completely different light. The scientist, in pursuit of fame, committed a deliberate deception, and the investigative authorities brought him to clean water, and thereby saved the health and lives of many. Whether this is true or not could be established based on the investigation materials.
But information that could shed light on everything related to the development of Perftoran is classified. If it becomes available, it will not be soon.
The story continues
Work on Perftoran resumed in 1991, when OJSC NPF Perftoran was created. The drug was launched into mass production. Several thousand patients received treatment with Perftoran. The majority had positive results. Sales initially increased sharply, but in 2015 they declined just as sharply.
This was due to insufficient funding and wear and tear of equipment. Although, perhaps the reason lies in the shortcomings of the product itself. After all, Perftoran can only be used in a hospital, and many doctors refuse it. The problem is not only the side effects, but also the high cost. But to replace blood, large volumes are needed, up to 20-30 ml per 1 kg of body weight.
Abroad, mainly in the USA, work also continues on the creation of blood substitutes based on PPO compounds. But none of these drugs have ever received approval for use from the US Food and Drug Administration.
And in Russia in 2021 they announced the creation of the latest generation emulsion PFD. The scope of application of perfluorocarbons is expanding. They can be used as preservatives for donor organs and tissues. These compounds may be useful in heart surgery.
The world's first human head transplant operation was announced for 2021. It was also planned to use perfluorocarbons during this process. True, the operation never took place.
Another area of application is cosmetology. Perfluorocarbons supply the skin and subcutaneous tissue with oxygen and prevent aging. Therefore, many cosmetic companies, incl. and Faberlic, known to many, included them in their products.
The question regarding the use of PFO emulsions as blood substitutes remains open. But it is already clear that not a single, even the most “advanced” compound, can replace what was created by Nature - natural blood. Perfluorocarbons transport oxygen but do not perform other functions in the blood. Therefore, the indications for their use should be reconsidered. And donated blood and drugs based on it will remain in demand in the future.
Author: Dotsenko Andrey Anatolyevich.