Pharmacodynamics and pharmacokinetics
The medicine is an antiulcer agent. It inhibits the action of H + -K + -ATPase in gastric parietal cells . Thus, the last stage of hydrochloric acid formation is blocked. The effect of the medicine depends on the dose. It effectively inhibits basal and stimulated secretions when used daily.
The drug reaches maximum effectiveness within 4 days. Its action in combination with antibacterial agents causes eradication of Helicobacter pylori . Due to this, the manifestations of the disease are stopped, stable remission is achieved, damage to the gastric mucosa is healed, and the risk of bleeding from the gastrointestinal tract is reduced. When using Losek Maps there is no need for long-term antiulcer therapy.
The tablets are absorbed in the small intestine. Absorption is completed 3-6 hours after consumption. When taking the product again, bioavailability increases to 60%. Eating has no effect on it. Plasma protein binding is approximately 95%.
The half-life from blood plasma is less than an hour. The active substance of the drug is completely broken down by the cytochrome , mainly in the liver. 80% of the drug is excreted in the form of metabolites in the urine, and another 20% in the feces.
Losek
Losek ®
(lat.
Losec ®
) is an original antiulcer drug, a proton pump inhibitor.
Active substance: omeprazole (lat. omeprazole
).
Losek is a global brand
Losec is the first proton pump inhibitor to enter the pharmaceutical market (USA) in 1989. Losec
sold in the United States was changed at the initiative of the FDA to
Prilosec
, but in a number of countries, including Russia, the
Losec
is still used today.
During its existence, Losek was produced and sold in various dosage forms. In the recent past, the most common form of Losec was capsules containing 10, 20 or 40 mg of omeprazole and taken orally. In 1998, a new dosage form was released - tablets made using MAPS technology (English MUPS - Multiple Unit Pellet System
). Each MAPS tablet contains from several hundred to several thousand acid-resistant microcapsules of omeprazole. This structure better protects the medicine from the aggressive environment of the stomach and ensures safer delivery of omeprazole to the small intestine, where omeprazole is absorbed into the blood.
Holding a patent on the MAPS form, AstraZeneca replaced the capsule form of Losec with MAPS tablets. Thus, in Russia, as well as in a number of other countries, oral forms of Losec are represented only by MAPS tablets, which are described in the article Losec MAPS
.
The only medicine sold in Russia
after 2004
under the Losek brand is a lyophilisate for the preparation of a solution for infusion.
Therefore, below the term “losec” will be understood only as this form of the drug.
By letter No. 20-3/408 of the Ministry of Health of the Russian Federation dated March 14, 2016, the registration of Losek (lyophilisate for the preparation of a solution for infusion) in Russia was canceled.
Losek composition
Losek for the preparation of a solution for infusion is a white or off-white lyophilisate, in the form of a pressed mass or powder.
One vial of Losec contains 42.6 mg of omeprazole sodium, which is equivalent to 40 mg of omeprazole. Excipients: 1.5 mg of disodium salt of ethylenediaminetetraacetic acid, sodium hydroxide 0.1–1.2 mg to obtain the required level of acidity, nitrogen, water for injection up to 2.0 ml.
Indications for use of Losek
Gastric and/or duodenal ulcers, reflux esophagitis, Zollinger-Ellison syndrome.
How to use Losek and dosage
Losek infusion is prescribed to patients with gastric and/or duodenal ulcers or reflux esophagitis who are unable to take tablets.
The recommended dose is 40 mg once daily. For patients with Zollinger-Ellison syndrome, doses are selected individually; initial intravenous administration of Losec is recommended at a dose of 60 mg per day. If the daily dose exceeds 60 mg, it should be divided into two doses. For patients with impaired renal function and the elderly, the dose is not adjusted. For patients with impaired liver function due to decreased release of omeprazole by the liver, a dose of 10–20 mg of omeprazole per day is usually sufficient.
Losec solution for infusion is recommended to be administered immediately after its preparation. The time for intravenous administration of Losek is 20–30 minutes.
Use of Losek during pregnancy and breastfeeding
. Losek does not have any side effects during pregnancy. for the fetus or newborn. During breastfeeding, omeprazole is excreted in breast milk, but there is no information about its effect on the child.
Contraindications to the use of Losek, warnings, pharmacological properties, interactions, etc.
- see article
omeprazole.
Professional medical materials regarding the use of Losek
- B.S. Briskin, H.S. Garcia. Possibilities of using the proton pump inhibitor omeprazole (Losec) for the treatment of bleeding duodenal ulcers. Pharmateka. – 2005. – No. 4–5 (100).
- Ilchenko A.A., Selezneva E.Ya., Dudik T.V. Clinical effectiveness of Losek MAPS and Losek for duodenal ulcer // Russian Journal of Gastroenterology, Hepatology, Coloproctology. –2001. – No. 4. – P. 29–32.
- Shapovalyants S.G., Mikhalev A.I., Babkova I.V. Modern pharmacotherapy of ulcerative gastroduodenal bleeding // Gastroenterology. 2021. Special issue No. 4.
- Manufacturer's instructions for medical use of the drug Losek (lyophilisate for the preparation of solution for infusion, for healthcare professionals, pdf)
On the website gastroscan.ru
in the literature catalog there is a section
“Omeprazole”
, containing medical articles concerning the treatment of diseases of the gastrointestinal tract with Losec and omeprazole.
Trade names of drugs with the active substance omeprazole
The following drugs are (were) registered in Russia: Bioprazole, Vero-Omeprazole, Gastrozol, Demeprazole, Zhelkizol, Zerotsid, Zolser, Chrismel, Lomak, Losek, Losek MAPS, Omegast, Omez, Omez Insta, Omecaps, Omepar, Omeprazole, Omeprazole pellets . , Pleom-20, Promez, Risek, Romesek, Sopral, Ulzol, Ulkozol, Ultop, Helitsid, Helol, Cisagast.
On the pharmaceutical markets of the countries of the former republics of the USSR, a number of drugs with the active substance omeprazole are presented, which are not registered in Russia, in particular: Gasek (Mepha Lda, Switzerland), Losid (Flamingo Pharmaceutical, India), Omeprazole-Astrapharm (TOV Astrapharm, Ukraine ), Omeprazole-Darnitsa (JSC Pharmaceutical Company Darnitsa, Ukraine), Omeprazole-KMP (JSC Kievmedpreparat, Ukraine), Omeprazole-Lugal (Lugansk Chemical Pharmaceutical Plant, Ukraine), Tserol (Neon Antibiotics Private Limited, India) and others. The branded medicine with the active ingredient omeprazole on the US and Canadian markets is Prilosec, the over-the-counter option is Prilosec OTC. In Germany, Italy and Switzerland, brands similar to Losek and Losek MAPS are called Antra and Antra MUPS ®. The manufacturer of Losec and other listed brands of omeprazole is AstraZeneca plc, UK. A number of major pharmaceutical companies have licenses to distribute Losek under their own name in certain countries.
Losek has contraindications, side effects and application features; consultation with a specialist is necessary.
Back to section
Indications for use
The medicine is prescribed for:
- acid-dependent dyspepsia;
- gastric ulcer caused by Helicobacter pylori;
- Zollinger-Ellison syndrome;
- peptic ulcer of the stomach and duodenum (including if the disease is caused by NSAIDs );
- gastroesophageal reflux.
Losek MAPS
Losek ® MAPS
(lat.
Losec ® MUPS ®
) is an original antiulcer drug, a proton pump inhibitor.
Active substance
:
omeprazole (lat. omeprazole
).
MAPS form of omeprazole
MAPS (eng. MUPS
- from
Multiple Unit Pellet System
) denotes a dosage form of omeprazole patented by AstraZeneca, in which each MAPS tablet consists of many microcapsules of magnesium omeprazole, coated with an additional protective coating. Thanks to this structure, omeprazole is not exposed to the aggressive effects of the acidic contents of the stomach, but when it enters the alkaline environment of the duodenum, it breaks down into omeprazole, which in turn is very quickly absorbed without loss, and magnesium, used by the body as an essential microelement (Shcherbakov P.L.) .
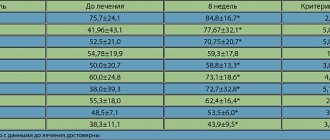
| Structure of MAPS tablets Losek MAPS (Maev I.V. et al.) |
MAPS tablets contain about 1000 acid-resistant microcapsules. The MAPS tablet, under the influence of gastric acid, disintegrates in the stomach into microcapsules protected from the acidic environment, then enters the small intestine, where, under the influence of alkaline acid, the microcapsules dissolve, omeprazole is released and absorbed. The MAPS form provides better delivery of omeprazole to the parietal cell, and as a result, a predictable and reproducible antisecretory effect. For erosive and ulcerative lesions of the gastroduodenal zone, MAPS tablets are as effective as omeprazole capsules. MAPS omeprazole can be dissolved in water or juice, which provides ease of use. The possibility of administering dissolved MAPS tablets through a nasogastric tube is especially relevant for seriously ill patients - the contingent of intensive care units, in whom the prevention of acute ulcers and erosions is an urgent task (Lapina T.L.).
Composition of Loseka MAPS
Losek, since 2005, has been supplied to Russia in two dosage forms: lyophilisate for preparing a solution for infusion (this drug is described in the article Losek
) and in the form of tablets made using MAPS technology. The shape of Losek MAPS tablets is biconvex, oblong, light pink, coated with printed omeprazole content. Two types of Losek MAPS tablets are supplied to Russia:
- containing 10.3 mg omeprazole magnesium, which is equivalent to 10 mg omeprazole
- containing 20.6 mg of omeprazole magnesium, which is equivalent to 20 mg of omeprazole.
Previously, 40 mg tablets were also supplied, which were red-brown in color.
Indications for use of Losec MAPS
Gastric and duodenal ulcers, including those associated with Helicobacter pylori, reflux esophagitis, Zollinger-Ellison syndrome, acid dyspepsia, NSAID-associated gastric ulcers or duodenal erosions.
In the treatment of diseases associated with Helicobacter pylori, Losek MAPS is used only as part of so-called “eradication regimens”, together with antibiotics.
Comparison of Losek MAPS with other drugs containing the active ingredient omeprazole
In terms of its pharmacological properties, Loseka MAPS does not differ from other PPIs with the active ingredient omeprazole.
The main advantage of Losek MAPS over other PPIs with the active ingredient omeprazole is that MAPS technology provides predictability regarding the entry of the required dose of omeprazole into the systemic circulation and, accordingly, greater confidence that the required therapeutic effect will be provided. Directions for use, dosage, pharmacological properties, etc. Loseka MAPS are determined by the active substance omeprazole and are described in the article “ Omeprazole
».
The main disadvantage of Losek MAPS is its high price, which is an order of magnitude higher than the price of such high-quality generics of omeprazole as Ultop, and higher than the original and also high-priced PPIs, such as Pariet (Maev I.V., etc.), is a thing of the past. The reduction in the cost of Losek MAPS in 2013 ensures greater availability of the original high-quality drug for patients (Maev I.V. et al., 2013).
There are many generics of omeprazole on the Russian market and the markets of other CIS countries. At the same time, if Losec MAPS, like other original PPIs, obviously has a high antisecretory potential, adequate in almost all situations with the need to suppress secretion, then generics are often inferior in antisecretory activity to original drugs. This is due to the quality of generics, as evidenced by the high “primary resistance” observed in certain drugs to the first standard doses, which decreases when the single dose is doubled (Kurilovich S.A., Chernosheikina L.E.).
Professional medical articles regarding the use of Losek MAPS
- Ivashkin V.T., Nemytin Yu.V., Makarov Yu.S. et al. Comparative assessment of the antisecretory activity of Losec MAPS, Pariet and Nexium in patients with peptic ulcer. TsVKG im. A.A. Vishnevsky, MMA named after. THEM. Sechenov.
- Ilchenko A.A., Selezneva E.Ya., Dudik T.V. Clinical effectiveness of Losek MAPS and Losek for duodenal ulcer // Russian Journal of Gastroenterology, Hepatology, Coloproctology. –2001. – No. 4. – P. 29–32.
- Lapina T.L. Treatment of erosive and ulcerative lesions of the stomach and duodenum // RMZh. – 2001. – volume 9. – No. 13–14. - With. 602–607.
- Maev I.V., Andreev D.N., Goncharenko A.Yu., Dicheva D.T. Proton pump inhibitors as the basis for the treatment of acid-related diseases // Handbook of a polyclinic physician. 2013. No. 7–8. pp. 12–14.
- Instructions for medical use of the drug Losek MAPS (film-coated tablets, 10 and 20 mg; pdf).
- Instructions (medical guide) for US patients from the manufacturer (in English, pdf): “Medication Guide Prilosec (omeprazole) Delayed-Release Capsules, Prilosec (omeprazole magnesium) for Delayed-Release Oral Suspensions.”
On the website gastroscan.ru in the literature catalog there is a section “Omeprazole”, containing medical articles concerning the treatment of diseases of the gastrointestinal tract with Losec and omeprazole.
Trade names of drugs with the active substance omeprazole
The following drugs are (were) registered in Russia: Bioprazole, Vero-Omeprazole, Gastrozol, Demeprazole, Zhelkizol, Zerotsid, Zolser, Chrismel, Lomak, Losek, Losek MAPS, Omegast, Omez, Omez Insta, Omecaps, Omepar, Omeprazole, Omeprazole pellets . , Pepticum, Pleom-20, Promez, Risek, Romesek, Sopral, Ulzol, Ulkozol, Ultop, Helitsid, Helol, Cisagast.
On the pharmaceutical markets of the countries of the former republics of the USSR, a number of drugs with the active substance omeprazole are presented, which are not registered in Russia, in particular: Gasek (Mepha Lda, Switzerland), Losid (Flamingo Pharmaceutical, India), Omeprazole-Astrapharm (TOV Astrapharm, Ukraine ), Omeprazole-Darnitsa (JSC Pharmaceutical Company Darnitsa, Ukraine), Omeprazole-KMP (JSC Kievmedpreparat, Ukraine), Omeprazole-Lugal (Lugansk Chemical Pharmaceutical Plant, Ukraine), Tserol (Neon Antibiotics Private Limited, India) and others.
The branded medicine with the active ingredient omeprazole on the US and Canadian markets is Prilosec (formerly called Losec), the over-the-counter option is Prilosec OTC. In Germany, Italy and Switzerland, analogues of Losek and Losek MAPS are sold under the brands Antra and Antra MUPS ®.
The manufacturer of Losek MAPS and the listed brands is AstraZeneca plc, UK/Sweden.
Losek MAPS has contraindications, side effects and application features; consultation with a specialist is necessary. Back to section
Side effects
As a rule, the medicine is well tolerated by patients. Undesirable side reactions are usually mild and pass quickly. Among them are:
- skin – rash, itching , photosensitivity, alopecia , erythema multiforme ;
- CNS – drowsiness , headache , vertigo , dizziness , paresthesia , insomnia , confusion , depression , agitation, hallucinations ;
- liver - liver dysfunction, increased levels of enzymes , encephalopathy (in liver diseases with complications), hepatitis ;
- circulatory system – leukopenia , agranulocytosis , thrombocytopenia , pancytopenia ;
- musculoskeletal system - muscle weakness , pain in joints and muscles;
- Gastrointestinal tract - diarrhea , pain in the abdominal area, vomiting, constipation , flatulence , dry mouth, candidiasis , stomatitis ;
- endocrine system – gynecomastia ;
- others - urticaria , fever , interstitial nephritis , angioedema , bronchospasm , anaphylactic shock , severe sweating , blurred vision, decreased sodium levels in the blood, peripheral edema , impaired taste, malaise .
Losek maps tablet p/o film 20mg vial plast 14 pcs
Inside. Losek® MAPS® tablets are recommended to be taken in the morning; the tablet should be swallowed whole with liquid. Tablets should not be chewed or crushed. The tablets can be dissolved in water or a slightly acidified liquid, such as fruit juice. The resulting solution must be used within 30 minutes. To ensure you take the full dose, fill the glass halfway with liquid again, shake and drink.
Adults
Duodenal ulcer
Patients with an active duodenal ulcer are recommended to take Losek® MAPS® 20 mg once a day. The drug provides rapid relief of symptoms. In most patients, ulcer healing occurs within 2 weeks. In cases where complete healing of the ulcer does not occur within 2 weeks, healing is achieved with a subsequent 2-week intake of the drug Losek® MAPS®.
Patients with duodenal ulcers that are poorly responsive to treatment are usually prescribed Losek® MAPS® 40 mg 1 time per day; Ulcer healing usually occurs within 4 weeks.
To prevent relapses, patients with duodenal ulcers are recommended Losek® MAPS® 10 mg 1 time per day. If necessary, the dose can be increased to 20-40 mg 1 time per day.
Stomach ulcer
The recommended dose is Losek® MAPS® 20 mg once a day. The drug provides rapid relief of symptoms. For most patients, cure occurs within 4 weeks. In cases where complete healing does not occur after the first course of taking the drug, a repeated 4-week course of treatment is usually prescribed, during which healing is achieved.
Patients with gastric ulcers that are poorly responsive to treatment are usually prescribed Losek® MAPS® 40 mg 1 time per day; healing is usually achieved within 8 weeks.
To prevent relapses, Losek® MAPS® 20 mg 1 time per day is recommended for patients with gastric ulcers. If necessary, the dose can be increased to 40 mg 1 time per day.
NSAID-associated ulcers and erosions of the stomach and duodenum
In the presence of NSAID-associated gastric, duodenal ulcers or gastroduodenal erosions in patients with ongoing NSAID therapy or after its cessation, the recommended dose of Losek® MAPS® is 20 mg 1 time per day. The drug provides rapid relief of symptoms; in most patients, cure occurs within 4 weeks. In those patients who do not heal during the initial period of therapy, healing is usually achieved with a repeat dose of the drug for 4 weeks.
For the prevention of ulcers and erosions of the stomach and duodenum and symptoms of dyspepsia associated with taking NSAIDs, the recommended dose of Losek® MAPS® is 20 mg 1 time per day.
Helicobacter pylori eradication in peptic ulcer (in combination with appropriate antibacterial therapy) Helicobacter pylori eradication regimens in peptic ulcer
Three-component treatment regimen:
Losek® MAPS® 20 mg, amoxicillin 1 g and clarithromycin 500 mg. Take all medications 2 times a day for one week or
Losec® MAPS® 20 mg, metronidazole 400 mg (or tinidazole 500 mg) and clarithromycin 250 mg. Take all medications 2 times a day for one week or
Losek® MAPS® 40 mg once a day, as well as amoxicillin 500 mg and metronidazole 400 mg 3 times a day for one week.
Two-component treatment regimen:
Losek® MAPS® 40-80 mg daily and amoxicillin 1.5 g daily (dose should be divided into parts) for two weeks. During clinical trials, amoxicillin was used in a daily dose of 1.5-3 g, Losek® MAPS® 40 mg once a day and clarithromycin 500 mg 3 times a day for two weeks.
To ensure complete healing, further treatment should be carried out in accordance with the recommendations in the sections “Duodenal Ulcer” and “Gastric Ulcer”.
In cases where, after completing a course of treatment, the test for Helicobacter pylori remains positive, the course of treatment can be repeated.
Reflux esophagitis
The recommended dose is one Losek® MAPS® 20 mg tablet once a day. The drug provides rapid relief of symptoms. For most patients, cure occurs within 4 weeks. In cases where complete cure does not occur after the first course of taking the drug, a repeated 4-week course of treatment is usually prescribed, during which a cure is achieved.
For patients with severe reflux esophagitis, Losek® MAPS® 40 mg 1 time per day is recommended; cure usually occurs within 8 weeks.
Patients with reflux esophagitis in remission are prescribed Losek® MAPS® 10 mg 1 time per day in the form of long courses of maintenance therapy. If necessary, the dose can be increased to 20-40 mg.
Symptomatic gastroesophageal reflux disease
The recommended dose is Losek® MAPS® 20 mg once a day. The drug provides rapid relief of symptoms. The therapeutic effect can be achieved with a daily dose of 10 mg, so individual dose selection cannot be ruled out. If after 4 weeks of treatment (Losec® MAPS® 20 mg 1 time per day) symptoms do not disappear, additional examination of the patient is recommended.
Dyspepsia associated with hyperacidity
To relieve pain and/or eliminate discomfort in the epigastric region, with or without heartburn, Losek® MAPS® 20 mg is prescribed once a day. The therapeutic effect can be achieved with a dose of 10 mg 1 time per day, so treatment can be started with this dose. If after 4 weeks of treatment (Losec® MAPS® 20 mg 1 time per day) symptoms do not disappear, additional examination of the patient is recommended.
Zollinger-Ellison syndrome
For patients with Zollinger-Ellison syndrome, the drug is prescribed in an individual dosage. Treatment is continued according to clinical indications for as long as necessary. The recommended starting dose is Losek® MAPS® 60 mg daily. In all patients with a severe form of the disease, as well as in cases where other therapeutic methods did not lead to the desired result, the use of the drug was effective in more than 90% of patients when taking 20-120 mg Losec® MAPS® daily. In cases where the daily dose of the drug exceeds 80 mg, the dose should be divided into two parts and taken 2 times a day.
Children and teenagers
Reflux esophagitis and symptomatic gastroesophageal reflux disease
Children over 2 years of age weighing more than 20 kg are prescribed Losek® MAPS® at a dose of 20 mg once a day. If necessary, the dose can be increased to 40 mg once a day. The recommended duration of treatment in case of reflux esophagitis is 4-8 weeks. The recommended duration of treatment for symptomatic gastroesophageal reflux disease is 2-4 weeks. If after 2-4 weeks of treatment control of the symptoms of the disease has not been achieved, additional examination of the patient is recommended.
Duodenal ulcer caused by Helicobacter pylori When choosing a treatment regimen, one should take into account official national, regional and local recommendations regarding the resistance of microorganisms to antibacterial agents, the duration of therapy (most often - 7 days, but in some cases - up to 14 days) and the correct use of antibacterial drugs drugs.
Therapy must be carried out under the supervision of a specialist.
For children over 4 years of age, the following treatment regimen is recommended: 15-30 kg - Losek® MAPS® 10 mg, amoxicillin 25 mg/kg and clarithromycin 7.5 mg/kg. Take all medications 2 times a day for a week. 31-40 kg - Losek® MAPS® 20 mg, amoxicillin 750 mg and clarithromycin 7.5 mg/kg. Take all medications 2 times a day for a week. > 40 kg - Losek® MAPS® 20 mg, amoxicillin 1 g and clarithromycin 500 mg. Take all medications 2 times a day for a week.
Special patient groups
Renal dysfunction
For patients with impaired renal function, no dose adjustment is required.
Liver dysfunction
In patients with impaired liver function, the bioavailability and plasma half-life of omeprazole are increased. In this regard, a dose of 10-20 mg per day is sufficient.
Elderly patients
For elderly patients, no dose adjustment is required.
Instructions for use Losek Maps (Method and dosage)
The instructions for use of Losec Maps tablets (and not Losec Max, as many believe) indicate that they are intended for oral use. Swallow whole without chewing. It is advisable to drink liquid. In addition, the tablets can be dissolved in fruit juice or water. This solution should be drunk within half an hour after preparation.
Instructions for use Losek Maps informs that the exact scheme of use of the medicine depends on the disease:
- stomach and duodenal ulcers in the acute phase - the patient should take 20 mg of the medicine every day. The course lasts 2 weeks. If complete scarring has not occurred, re-therapy can be carried out for another 2 weeks;
- the need to prevent exacerbation of duodenal ulcer - the patient should take 10 mg of medication every day. If necessary, the dosage is increased to 20-40 mg;
- stomach ulcer resistant to therapy - 40 mg of medication per day. Recovery is usually within eight weeks;
- erosive and ulcerative lesions in the gastrointestinal tract (gastroduodenal department) in HIV-infected patients - 20 mg is used once a day. Compatible with HIV therapy. The course lasts on average 4 weeks. If complete cure has not occurred, therapy can be repeated;
- stomach ulcer caused by Helicobacter pylori - three different treatment regimens are possible. 1) 20 mg Losec Maps, 500 mg Clarithromycin and 1 g Amoxicillin . 2) 20 mg Losec Maps, 250 mg Clarithromycin and 500 mg Tinidazole (or 400 mg Metronidazole ). All medications must be taken 2 times daily for a week. 3) For a week, take every day 20 mg Losec Maps 1 time, 400 mg Metronidazole 3 times, 500 mg Amoxicillin 3 times. In addition, the doctor may prescribe “dual therapy.” In this case, Losek Maps is drunk daily in a dosage of 40-80 mg, and Amoxicillin - 1.5 g (the dose is divided into parts). The course lasts two weeks. After eradication of Helicobacter pylori, subsequent therapy is carried out according to the usual regimen, as for duodenal and gastric ulcers . If at the end of the course the test for Helicobacter pylori is positive, treatment can be repeated;
- duodenal ulcer resistant to therapy - 40 mg of medication daily. Healing in most cases occurs within a month;
- stomach ulcer in the acute phase - you need to take 20 mg of medication per day. Therapy is designed for 4 weeks. If the ulcer has not completely healed at the end of the course, treatment can be repeated;
- the need to prevent exacerbations of stomach ulcers - you should take 20 mg of medication per day. If necessary, the daily dosage is increased to 40 mg;
- the need to prevent exacerbations of gastric and duodenal ulcers , the appearance of dyspeptic symptoms due to gastric ulcers , as well as erosive lesions of the gastroduodenal region - experts prescribe patients to take 20 mg of medication daily;
- esophageal reflux – 20 mg of medication per day. Therapy lasts an average of a month. If the patient is not completely cured, the course can be repeated;
- esophageal reflux in remission - experts prescribe taking 10 mg of Losec Maps per day, if necessary, the dose can be increased to 20-40 mg. The appointment is designed for a long course of maintenance therapy;
- pain, discomfort and heartburn in the epigastric region associated with acid-dependent dyspepsia - start with a daily dosage of 10 mg, which, if necessary, can be increased to 20 mg. Therapy is designed for 4 weeks. If the symptoms do not disappear upon completion of the course, it is advisable to change the treatment regimen;
- severe form of esophageal reflux - 40 mg of medication per day. Therapy is usually designed for 2 months;
- symptomatic gastroesophageal reflux - dosages are determined in each case individually. Usually take 10-20 mg daily. Therapy is designed for 4 weeks. If the symptoms have not disappeared at the end of the treatment, it is advisable to change the Losek Maps treatment regimen;
- Zollinger-Ellison syndrome - dosages are individual in each case. The recommended dose to start therapy is 60 mg every day. If the daily dosage is more than 80 mg, it must be divided into 2 doses.
If the patient has problems with liver function, the bioavailability and clearance of the active substance Losec Maps increases, so the daily dosage should usually not exceed 20 mg.
Losec MAPS, 14 pcs., 20 mg, film-coated tablets
Inside, in the morning, the tablet should be swallowed whole, without chewing, with liquid (you can dissolve the tablet in water or a slightly acidified liquid, such as fruit juice; the resulting solution should be used within 30 minutes).
Duodenal ulcer: in the acute phase - 20 mg 1 time per day daily for 2 weeks; if there is no effect within 2 weeks, a repeat 2-week course is prescribed. For duodenal ulcer resistant to therapy - 40 mg once a day for 4 weeks. To prevent relapses of duodenal ulcer - 10 mg (if necessary - up to 20-40 mg/day) 1 time per day.
Gastric ulcer: 20 mg 1 time per day daily for 4 weeks; if there is no effect, repeat the 4-week course. For gastric ulcers resistant to therapy - 40 mg once a day for 8 weeks. To prevent relapses of gastric ulcer - 20 mg (if necessary - up to 40 mg) 1 time per day.
NSAID-associated gastric ulcers or duodenal erosions: 20 mg once a day for 4 weeks. If there is no effect, a repeat 4-week course of treatment is prescribed.
Helicobacter pylori eradication regimens for gastric ulcers.
Three-component treatment regimen:
Losec MAPS 20 mg, amoxicillin 1 g and clarithromycin 500 mg (take all drugs 2 times a day for 1 week) or Losec MAPS 20 mg, metronidazole 400 mg (or tinidazole 500 mg) and clarithromycin 250 mg (take all drugs 2 times per day for 1 week), or Losek MAPS 40 mg 1 time per day, amoxicillin 500 mg and metronidazole 400 mg 3 times per day for 1 week.
Two-component treatment regimen: Losec MAPS 40–80 mg and amoxicillin 1.5 g daily (the dose should be divided into parts) for 2 weeks. During clinical trials, Losec MAPS 40 mg was used once a day, amoxicillin in a daily dose of 1.5–3 g and clarithromycin 500 mg 3 times a day for 2 weeks. If after treatment the test for Helicobacter pylori remains positive, the course can be repeated.
Reflux esophagitis: 20 mg once a day for 4 weeks. If there is no effect, repeat the 4-week course. For patients with severe reflux esophagitis - 40 mg once a day for 8 weeks. For reflux esophagitis in remission - 10 mg once a day (if necessary - up to 20-40 mg/day) for a long time.
Symptomatic gastroesophageal reflux disease, dyspepsia associated with increased production of hydrochloric acid: 10–20 mg/day daily for 4 weeks. If symptoms do not disappear at the end of therapy, additional examination of the patient is recommended.
Zollinger-Ellison syndrome: the dosage regimen is determined individually. The recommended starting dose is 60 mg/day. In all patients with severe disease, as well as in cases where other therapeutic methods did not lead to the desired result, the drug was effective in more than 90% of patients when taking 20-120 mg of Losec MAPS daily. If the daily dose exceeds 80 mg, it should be divided into 2 hours and taken 2 times a day.
In elderly patients, as well as in patients with impaired renal function, no dose adjustment is required.
If liver function is impaired, the bioavailability of omeprazole and T1/2 from plasma increases; therefore, a dose of 10–20 mg is sufficient.
Interaction
The combination of Losec Maps and Ketoconazole may lead to a decrease in the degree of absorption of the latter.
In addition, the drug may increase the elimination time of Warfarin , Phenytoin and Diazepam . Therefore, dosage adjustments may be necessary. But daily use of 20 mg Losec Maps does not affect the level of Phenytoin in the blood plasma during long-term therapy, and also does not change the coagulation during long-term use of Warfarin .
The combined use of omeprazole and Clarithromycin causes an increase in their plasma concentrations.
Instructions for use LOSEC
The effect of omeprazole on the pharmacokinetics of other drugs
Reduced acidity in the stomach during treatment with omeprazole can lead to a decrease or increase in the absorption of other drugs, the mechanism of absorption of which depends on the acidity of the environment. As with other drugs that suppress the secretion of hydrochloric acid or antacids, treatment with omeprazole may lead to decreased absorption of ketoconazole or itraconazole, as well as increased absorption of digoxin. Combined use of omeprazole at a dose of 20 mg once a day. and digoxin increases the bioavailability of digoxin by 10% (bioavailability of digoxin increased by up to 30% in 20% of patients).
Omeprazole has been shown to interact with some antiretroviral drugs. The mechanisms and clinical significance of these interactions are not always known. An increase in pH during omeprazole therapy may affect the absorption of antiretroviral drugs. Interaction at the level of the CYP2C19 isoenzyme is also possible. When omeprazole is co-administered with certain antiretroviral drugs, such as atazanavir and nelfinavir, during therapy with omeprazole, a decrease in their serum concentrations is observed. In this regard, the combined use of omeprazole with antiretroviral drugs such as atazanavir and nelfinavir is not recommended.
With the simultaneous use of omeprazole and saquinavir, an increase in the concentration of saquinavir in the serum was noted; when used with some other antiretroviral drugs, their concentration did not change.
Omeprazole inhibits CYP2C19, the main isoenzyme involved in its metabolism. Concomitant use of omeprazole with other drugs that are metabolized by the CYP2C19 isoenzyme, such as diazepam, phenytoin, warfarin, other vitamin K antagonists and cilostazol, may lead to a decrease in the metabolism of these drugs.
It is recommended to monitor plasma phenytoin concentrations when taking phenytoin and omeprazole together; in some cases it may be necessary to reduce the dose of phenytoin. At the same time, in patients taking phenytoin for a long time, co-administration of omeprazole at a dose of 20 mg 1 time / day did not cause changes in the concentration of phenytoin in the blood plasma.
When using omeprazole in patients receiving warfarin or other vitamin K antagonists, monitoring of the MHO index (international normalized ratio) is necessary; in some cases, it may be necessary to reduce the dose of warfarin or another vitamin K antagonist. At the same time, in patients taking warfarin for a long time. joint use of omeprazole at a dose of 20 mg 1 time/day did not cause a change in coagulation time.
The use of omeprazole at a dose of 40 mg 1 time / day led to an increase in Cmax and AUC of cilosgazole by 18% and 26%, respectively; for one of the active metabolites of cilostazol, the increase was 29% and 69%, respectively.
Omeprazole does not affect the metabolism of drugs that are metabolized by the CYP3A4 isoenzyme, such as cyclosporine, lidocaine, hipidine, estradiol, erythromycin and budesonide.
There was no effect of omeprazole on the following drugs:
- caffeine, theofidline, quinidine, piroxicam, diclofenac, naproxen, metoprolol, propranolol and ethanol.
With the simultaneous use of omeprazole and tacrolimus, an increase in the concentration of tacrolimus in the blood serum was noted.
Effect of drugs on the pharmacokinetics of omeprazole
The isoenzymes CYP2C19 and CYP3A4 are involved in the metabolism of omeprazole. The combined use of omeprazole and inhibitors of the CYP2C19 and CYP3A4 isoenzymes, such as clarithromycin and voriconazole, may lead to an increase in the concentration of omeprazole in the blood plasma by slowing down the metabolism of omeprazole. Concomitant use of voriconazole and omeprazole results in a more than twofold increase in the AUC value for omeprazole. Due to the good tolerance of high doses of omeprazole, short-term co-administration of these drugs does not require dose adjustment of omeprazole.
Drugs that induce CYP2C19 and CYP3A4 isoenzymes, such as rifampicin and St. John's wort preparations, when used together with omeprazole, may lead to a decrease in the concentration of omeprazole in the blood plasma by accelerating the metabolism of omeprazole.
Effect on clopidogrel therapy
During therapy with omeprazole, the antithrombotic effect of clopidogrel may be reduced. The mechanism of this interaction has not yet been fully studied, so it is recommended to use these drugs together with caution.
Analogues of Losek Maps
Level 4 ATC code matches:
Khairabesol
Noflux
Lancid
Barol
Beret
Ontime
Gastrozol
Omeprazole
Pantoprazole
Proxium
Lansoprazole
Zulbex
Ultop
Epicurus
Pariet
Losek
Sanpraz
Emanera
Omez Insta
Omez
The most common analogues of Losek Maps:
- Ultop;
- Losek;
- Helicid;
- Omeprazole;
- Omitox.
Each of these medications has its own nuances of use, so you should not take them on your own, without a doctor’s prescription.
Losek maps 20 mg 28 pcs. film-coated tablets
pharmachologic effect
Gastric gland secretion inhibitor, proton pump inhibitor.
Composition and release form Losek maps 20 mg 28 pcs. film-coated tablets
Tablets - 1 tablet:
- Active substance: magnesium omeprazole 20.6 mg (equivalent to omeprazole 20.0 mg);
- Excipients: glyceryl monostearate 40-55 1.4 mg, hyprolose 4.8 mg, hypromellose 15.0 mg, magnesium stearate 0.7 mg, methacrylic and ethacrylic acid copolymer 27.0 mg, microcrystalline cellulose 220.0 mg, paraffin 0.2 mg, macrogol 2.5 mg, polysorbate 80 0.1 mg, crospovidone 4.6 mg, sodium stearyl fumarate 0.5 mg, sucrose;
- Shell: talc 8.3 mg, titanium dioxide (E171) 2.2 mg, triethyl citrate 8.2 mg, iron dye red oxide E172 0.3 mg.
14 or 28 tablets in a bottle made of high-density polyethylene with a screw cap made of polypropylene, equipped with a protective ring that ensures first opening control, and a desiccant capsule mounted inside the cap.
1 bottle with instructions for use in a cardboard box with first opening control.
Description of the dosage form
An oblong, biconvex, pink to light brownish-pink, film-coated tablet, debossed with 20 mG on one side and the symbol on the other side.
Characteristic
10 mg tablets: light pink, biconvex, oblong, coated, with 10 mg engraved on one side and the company logo on the other.
20 mg tablets: pink, biconvex, oblong, coated, with 20 mg engraved on one side and the company logo on the other.
40 mg tablets: red-brown, biconvex, oblong, coated, with 40 mg engraved on one side and the company logo on the other.
Directions for use and doses
Inside. Losek® MAPS® tablets are recommended to be taken in the morning; the tablet should be swallowed whole with liquid. Tablets must not be chewed or crushed.
The tablets can be dissolved in water or a slightly acidified liquid, such as fruit juice. The resulting solution must be used within 30 minutes. To make sure you take the full dose, fill the glass halfway with liquid again, shake and drink.
Adults
Duodenal ulcer
Patients with an active duodenal ulcer are recommended to take Losek® MAPS® 20 mg once a day. The drug provides rapid relief of symptoms. In most patients, ulcer healing occurs within 2 weeks. In cases where complete healing of the ulcer does not occur within 2 weeks, healing is achieved with a subsequent 2-week intake of the drug Losek® MAPS®.
Patients with duodenal ulcers that are poorly responsive to treatment are usually prescribed Losek® MAPS® 40 mg 1 time per day; Ulcer healing usually occurs within 4 weeks.
To prevent relapses, patients with duodenal ulcers are recommended Losek® MAPS® 10 mg once a day. If necessary, the dose can be increased to 20-40 mg 1 time per day.
Stomach ulcer
The recommended dose is Losek® MAPS® 20 mg once a day. The drug provides rapid relief of symptoms. For most patients, cure occurs within 4 weeks. In cases where complete healing does not occur after the first course of taking the drug, a repeated 4-week course of treatment is usually prescribed, during which healing is achieved.
Patients with gastric ulcers that are poorly responsive to treatment are usually prescribed Losek® MAPS® 40 mg 1 time per day; healing is usually achieved within 8 weeks.
To prevent relapses, patients with gastric ulcers are recommended Losek® MAPS® 20 mg once a day. If necessary, the dose can be increased to 40 mg 1 time per day. NSAID-associated ulcers and erosions of the stomach and duodenum
In the presence of NSAID-associated gastric, duodenal ulcers or gastroduodenal erosions in patients with ongoing NSAID therapy or after its cessation, the recommended dose of Losek® MAPS® is 20 mg 1 time per day. The drug provides rapid relief of symptoms; in most patients, cure occurs within 4 weeks. In those patients who do not heal during the initial period of therapy, healing is usually achieved with a repeat dose of the drug for 4 weeks.
For the prevention of ulcers and erosions of the stomach and duodenum and symptoms of dyspepsia associated with taking NSAIDs, the recommended dose of Losek® MAPS® is 20 mg 1 time per day.
Eradication of Helicobacter pylori in peptic ulcer disease (in combination with appropriate antibacterial therapy)
Helicobacter pylori eradication regimens for peptic ulcer disease
Three-component treatment regimen:
Losek® MAPS® 20 mg, amoxicillin 1 g and clarithromycin 500 mg. Take all medications 2 times a day for one week
or
Losek® MAPS® 20 mg. metronidazole 400 mg (or tinidazole 500 mg) and clarithromycin 250 mg. Take all medications 2 times a day for one week
or
Losek® MAPS® 40 mg once a day, as well as amoxicillin 500 mg and metronidazole 400 mg 3 times a day for one week.
Two-component treatment regimen:
Losek® MAPS® 40-80 mg daily and amoxicillin 1.5 g daily (dose should be divided into parts) for two weeks. During clinical trials, amoxicillin was used in a daily dose of 1.5-3 g, Losek® MAPS® 40 mg once a day and clarithromycin 500 mg 3 times a day for two weeks.
To ensure complete healing, further treatment should be carried out in accordance with the recommendations in the sections “Duodenal Ulcer” and “Gastric Ulcer”.
In cases where, after completing a course of treatment, the test for Helicobacter pylori remains positive, the course of treatment can be repeated.
Reflux esophagitis
The recommended dose is one Losek® MAPS® 20 mg tablet once a day. The drug provides rapid relief of symptoms. For most patients, cure occurs within 4 weeks. In cases where complete cure does not occur after the first course of taking the drug, a repeated 4-week course of treatment is usually prescribed, during which a cure is achieved.
For patients with severe reflux esophagitis, Losek® MAPS® 40 mg 1 time per day is recommended; cure usually occurs within 8 weeks.
Patients with reflux esophagitis in remission are prescribed Losek® MAPS® 10 mg 1 time per day as long-term courses of maintenance therapy. If necessary, the dose can be increased to 20-40 mg.
Symptomatic gastroesophageal reflux disease
The recommended dose is Losek® MAPS® 20 mg once a day. The drug provides rapid relief of symptoms. The therapeutic effect can be achieved with a daily dose of 10 mg, so individual dose selection cannot be ruled out. If after 4 weeks of treatment (Losec® MAPS® 20 mg 1 time per day) symptoms do not disappear, additional examination of the patient is recommended.
Dyspepsia associated with hyperacidity
To relieve pain and/or eliminate discomfort in the epigastric region, with or without heartburn, Losek® MAPS® 20 mg is prescribed once a day. The therapeutic effect can be achieved with a dose of 10 mg 1 time per day, so treatment can be started with this dose. If after 4 weeks of treatment (Losec® MAPS® 20 mg 1 time per day) symptoms do not disappear, additional examination of the patient is recommended.
Zollinger-Ellison syndrome
For patients with Zollinger-Ellison syndrome, the drug is prescribed in an individual dosage. Treatment is continued according to clinical indications for as long as necessary. The recommended starting dose is Losec® MAPS® 60 mg daily. In all patients with a severe form of the disease, as well as in cases where other therapeutic methods did not lead to the desired result, the use of the drug was effective in more than 90% of patients when taking 20-120 mg Losec® MAPS® daily. In cases where the daily dose of the drug exceeds 80 mg, the dose should be divided into two parts and taken 2 times a day.
Children and teenagers
Reflux esophagitis and symptomatic gastroesophageal reflux disease
Children over the age of 2 years with a body weight of more than 20 kg are prescribed the drug Losek® MAPS® at a dose of 20 mg once a day. If necessary, the dose can be increased to 40 mg once a day. The recommended duration of treatment in case of reflux esophagitis is 4-8 weeks. The recommended duration of treatment for symptomatic gastroesophageal reflux disease is 2-4 weeks. If after 2-4 weeks of treatment control of the symptoms of the disease has not been achieved, additional examination of the patient is recommended.
Duodenal ulcer caused by Helicobacter pylori
When choosing a treatment regimen, official national, regional and local recommendations should be taken into account. concerning the resistance of microorganisms to antibacterial agents, the duration of therapy (most often - 7 days, but in some cases - up to 14 days) and the correct use of antibacterial drugs.
Therapy must be carried out under the supervision of a specialist.
For children over 4 years of age, the following treatment regimen is recommended:
- Body weight 15-30 kg - Losek® MAPS® 10 mg, amoxicillin 25 mg/kg and clarithromycin 7.5 mg/kg. Take all medications 2 times a day for a week.
- Body weight 31 -40 kg - Losek® MAPS® 20 mg, amoxicillin 750 mg and clarithromycin 7.5 mg/kg. Take all medications 2 times a day for a week.
- Body weight > 40 kg - Losek® MAPS® 20 mg, amoxicillin 1 g and clarithromycin 500 mg. Take all medications 2 times a day for a week.
Special patient groups
Renal dysfunction
For patients with impaired renal function, no dose adjustment is required.
Liver dysfunction
In patients with impaired liver function, the bioavailability and plasma half-life of omeprazole are increased. In this regard, a dose of 10-20 mg per day is sufficient.
Elderly patients
For elderly patients, no dose adjustment is required.
Pharmacodynamics
Mechanism of action
Omeprazole is a weak base. Concentrated in the acidic environment of the secretory tubules of the parietal cells of the gastric mucosa, it is activated and inhibits the proton pump - the enzyme H+, K+-ATPase. The effect of omeprazole on the last stage of the formation of hydrochloric acid in the stomach is dose-dependent and provides highly effective inhibition of basal and stimulated secretion of hydrochloric acid, regardless of the stimulating factor.
Effect on gastric juice secretion
Losek® MLPS®, when administered daily orally, provides rapid and effective inhibition of daytime and nighttime hydrochloric acid secretion. The maximum effect is achieved within 4 days of treatment. In patients with duodenal ulcers, Losek® MAPS® 20 mg causes a sustained decrease in 24-hour gastric acidity by at least 80%. In this case, a decrease in the average maximum concentration of hydrochloric acid after stimulation with pentagastrin by 70% is achieved within 24 hours.
In patients with duodenal ulcers, Losek® MAPS® 20 mg, with daily oral administration, maintains an acidity value in the intragastric environment at a pH level of ≥ 3, on average, for 17 hours a day.
Inhibition of hydrochloric acid secretion depends on the area under the concentration-time curve (AUC) of omeprazole, and not on the plasma concentration of the drug at a given time.
Effect on Helicobacter pylori
Omeprazole has a bactericidal effect against Helicobacter pylori in vitro. Eradication of Helicobacter pylori when using omeprazole in conjunction with antibacterial agents is accompanied by rapid elimination of symptoms, a high degree of healing of defects in the mucous membrane of the gastrointestinal tract and long-term remission of peptic ulcer disease, which reduces the likelihood of complications such as bleeding, as effectively as continuous maintenance therapy.
Other effects associated with inhibition of hydrochloric acid secretion
Patients taking drugs that reduce the secretion of gastric glands for a long period of time are more likely to experience the formation of glandular cysts in the stomach; The cysts are benign and go away on their own with continued therapy. These phenomena are caused by physiological changes resulting from inhibition of hydrochloric acid secretion.
Reducing the secretion of hydrochloric acid in the stomach under the influence of proton pump inhibitors or other agents that reduce gastric acidity leads to an increase in the growth of normal intestinal microflora, which in turn may lead to a slight increase in the risk of developing intestinal infections caused by bacteria of the genus Salmonella spp. and Campylobacter spp., and in hospitalized patients, probably also Clostridium difficile.
During treatment with drugs that reduce the secretion of gastric glands, the concentration of gastrin in the blood serum increases. Due to decreased secretion of hydrochloric acid, the concentration of chromogranin A (CgA) increases. An increase in CgA concentration may affect the results of examinations to detect neuroendocrine tumors (see section "Special Instructions"). To prevent this effect, therapy with proton pump inhibitors must be suspended at least 5 days before testing CgA concentrations. If CgA and gastrin concentrations have not returned to normal during this time, the study should be repeated 14 days after stopping omeprazole.
In children and adult patients taking omeprazole for a long time, an increase in the number of enterochromaffin-like cells was observed, probably associated with an increase in the concentration of gastrin in the blood serum. This phenomenon has no clinical significance.
Pharmacokinetics
Distribution
Omeprazole is absorbed in the small intestine, usually within 3-6 hours. Bioavailability after oral administration is approximately 60%. Food intake does not affect the bioavailability of omeprazole.
The binding rate of omeprazole to plasma proteins is about 95%. the volume of distribution is 0.3 l/kg.
Metabolism
Omeprazole is completely metabolized in the liver. The main enzymes involved in the metabolic process. CYP2C19 and CYP3A4. The resulting metabolites - sulfone, sulfide and hydroxyomeprazole do not have a significant effect on the secretion of hydrochloric acid.
The total plasma clearance is 0.3-0.6 l/min. The bioavailability of omeprazole increases by approximately 50% with repeated doses compared to a single dose.
Excretion
The half-life is approximately 40 minutes (30-90 minutes). About 80% is excreted as metabolites by the kidneys, and the rest by the intestines.
Special patient groups
There were no significant changes in the bioavailability of omeprazole in elderly patients or in patients with impaired renal function. In patients with impaired liver function, there is an increase in the bioavailability of omeprazole and a significant decrease in plasma clearance.
Indications for use Losek maps 20 mg 28 pcs. film-coated tablets
Adults:
- duodenal ulcer;
- stomach ulcer;
- NSAID-associated ulcers and erosions of the stomach and duodenum;
- eradication of Helicobacter pylori in peptic ulcer disease (in combination with appropriate antibacterial therapy);
- reflux esophagitis;
- symptomatic gastroesophageal reflux disease;
- dyspepsia associated with high acidity;
- Zollinger-Ellison syndrome.
Children and teenagers
Children over 2 years old weighing at least 20 kg:
- reflux esophagitis;
- symptomatic gastroesophageal reflux disease.
Children over 4 years old and teenagers:
- Duodenal ulcer caused by Helicobacter pylori.
Contraindications
- Known hypersensitivity to omeprazole, substituted benzimidazoles or other ingredients included in the drug.
- Sucrase/isomaltase deficiency, fructose intolerance, glucose-galactose malabsorption.
- Concomitant use with erlotinib, posaconazole.
- Children under 2 years of age.
- Children over 2 years of age for indications other than the treatment of reflux esophagitis and symptomatic gastroesophageal reflux disease.
- Children over 4 years of age for indications other than the treatment of reflux esophagitis, symptomatic gastroesophageal reflux disease and duodenal ulcers caused by Helicobacter pylori.
Carefully:
Patients with osteoporosis.
If symptoms such as significant spontaneous weight loss, frequent vomiting, dysphagia, haematemesis or melena are present, or if a gastric ulcer is present (or a gastric ulcer is suspected), malignancy should be excluded as treatment may mask symptoms and , thus delaying diagnosis.
Application of Losek maps 20 mg 28 pcs. film-coated tablets during pregnancy and breastfeeding
The research results showed no side effects of omeprazole on the health of pregnant women, the fetus or the newborn.
Losek® MAPS® can be used during pregnancy.
Omeprazole passes into breast milk, however, when used in therapeutic doses, exposure to the child is unlikely.
special instructions
If any alarming symptoms are present (eg, significant spontaneous weight loss, repeated vomiting, dysphagia, hematemesis, or melena), or if a gastric ulcer is present (or if a gastric ulcer is suspected), malignancy should be excluded because Treatment with Losek® MAPS® can lead to a smoothing of symptoms and delay diagnosis.
The combined use of omeprazole with drugs such as atazanavir and nelfinavir is not recommended.
According to the study results, a pharmacokinetic/pharmacodynamic interaction was noted between clopidogrel (loading dose of 300 mg and maintenance dose of 75 mg/day) and omeprazole (80 mg/day orally), which leads to a decrease in exposure to the active metabolite of clopidogrel by an average of 46% and reducing the maximum inhibition of ADP-induced platelet aggregation by an average of 16%. Therefore, the simultaneous use of omeprazole and clopidogrel should be avoided.
Individual observational studies indicate that proton pump inhibitor therapy may modestly increase the risk of osteoporosis-related fractures, but other similar studies have not reported an increased risk.
Randomized, double-blind, controlled clinical trials of omeprazole and esomeprazole, including two open-label studies with treatment durations of more than 12 years, did not confirm the association of osteoporotic fractures with the use of proton pump inhibitors.
Although a causal relationship between the use of omeprazole/esomeprazole and osteoporotic fractures has not been established, patients at risk of developing osteoporosis or osteoporotic fractures should be under appropriate clinical supervision.
Increased concentrations of CgA may affect the results of examinations for the detection of neuroendocrine tumors. To prevent this effect, therapy with proton pump inhibitors must be suspended at least 5 days before testing CgA concentrations. If CgA and gastrin concentrations have not returned to normal during this time, the study should be repeated 14 days after stopping omeprazole.
Impact on the ability to drive vehicles and operate machinery
There is no data on the effect of the drug Losek® MAPS® on the ability to drive vehicles and machines. However, due to the fact that dizziness, blurred vision and drowsiness may occur during therapy, caution should be exercised when driving vehicles and machinery.
Overdose
Single oral doses of Losec® MAPS® up to 400 mg did not cause any severe symptoms. When adults took 560 mg of omeprazole, moderate intoxication was observed. As the dose was increased, the rate of drug elimination did not change (first-order kinetics), and no specific treatment was required.
Symptoms: dizziness, confusion, apathy, headache, vascular dilatation, tachycardia, nausea, vomiting, flatulence, diarrhea.
Treatment: symptomatic treatment, gastric lavage if necessary, administration of activated charcoal.
Side effects Losek maps 20 mg 28 pcs. film-coated tablets
The following are side effects, independent of the dosage regimen of omeprazole, that were noted during clinical studies, as well as during post-marketing use.
Often (>1/100,
Uncommon (>1/1000,
Rarely (>1/10000,
Not known: Hypomagnesemia, hypocalcemia due to severe hypomagnesemia, hypokalemia due to hypomagnesemia.
Cases of the formation of glandular cysts in the stomach have been reported in patients taking drugs that reduce the secretion of gastric glands for a long period of time; The cysts are benign and go away on their own with continued therapy.
Drug interactions
The effect of omeprazole on the pharmacokinetics of other drugs
A decrease in the secretion of hydrochloric acid in the stomach during treatment with omeprazole and other proton pump inhibitors can lead to a decrease or increase in the absorption of other drugs, the absorption of which depends on the acidity of the environment.
Like other drugs that reduce gastric acidity, treatment with omeprazole may result in decreased absorption of ketoconazole, itraconazole, posaconazole and erlotinib, and increased absorption of drugs such as digoxin. Co-administration of omeprazole 20 mg once daily and digoxin increases the bioavailability of digoxin by 10% (the bioavailability of digoxin increased by up to 30% in 20% of patients). Concomitant use of Losec® MAPS® with erlotinib or posaconazole is contraindicated (see section “Contraindications”). Omeprazole has been shown to interact with some antiretroviral drugs. The mechanisms and clinical significance of these interactions are not always known. An increase in pH during omeprazole therapy may affect the absorption of antiretroviral drugs. Interaction at the level of the CYP2C19 isoenzyme is also possible. When omeprazole is co-administered with certain antiretroviral drugs, such as atazanavir and nelfinavir, a decrease in their serum concentrations is observed during omeprazole therapy. In this regard, the combined use of omeprazole with antiretroviral drugs such as atazanavir and nelfinavir is not recommended.
With the simultaneous use of omeprazole and saquinavir, an increase in the concentration of saquinavir in the serum was noted; when used with some other antiretroviral drugs, their concentration did not change.
Omeprazole inhibits CYP2C19, the main isoenzyme involved in its metabolism. Concomitant use of omeprazole with other drugs metabolized by the CYP2C19 isoenzyme, such as diazepam, warfarin (R-warfarin) or other vitamin K antagonists, phenytoin and cilostazol, may lead to a slower metabolism of these drugs. Monitoring of patients taking phenytoin and omeprazole is recommended; a dose reduction of phenytoin may be required. However, concomitant treatment with omeprazole at a daily dose of 20 mg does not affect the concentration of phenytoin in the blood plasma in patients taking the drug for a long time. When using omeprazole in patients receiving warfarin or other vitamin K antagonists, monitoring of the international normalized ratio is necessary; in some cases, it may be necessary to reduce the dose of warfarin or another vitamin K antagonist. At the same time, concomitant treatment with omeprazole at a daily dose of 20 mg does not lead to a change in coagulation time in patients taking warfarin for a long time.
The use of omeprazole at a dose of 40 mg once daily led to an increase in Cmax and AUC of cilostazol by 18% and 26%, respectively; for one of the active metabolites of cilostazol, the increase was 29% and 69%, respectively.
According to the study results, a pharmacokinetic/pharmacodynamic interaction was noted between clopidogrel (loading dose of 300 mg and maintenance dose of 75 mg/day) and omeprazole (80 mg/day orally), which leads to a decrease in exposure to the active metabolite of clopidogrel by an average of 46 % and reducing the maximum inhibition of ADP-induced platelet aggregation by an average of 16%.
The clinical significance of this interaction is unclear. An increased risk of cardiovascular events with concomitant use of clopidogrel and proton pump inhibitors, including omeprazole, was not shown in a prospective, randomized, open-label study of more than 3,760 patients receiving placebo or omeprazole 20 mg/day. concomitantly with clopidogrel and acetylsalicylic acid (ASA) therapy, and was not confirmed by additional non-randomized analysis of clinical outcomes from large prospective randomized trials involving more than 47,000 patients.
The results of a number of observational studies are contradictory and do not provide a clear answer about the presence or absence of an increased risk of thromboembolic cardiovascular complications during the combined use of clopidogrel and proton pump inhibitors.
When clopidogrel was used together with a fixed combination of 20 mg esomeprazole and 81 mg ASA, exposure to the active metabolite of clopidogrel decreased by almost 40% compared with clopidogrel monotherapy, while the maximum levels of inhibition of ADP-induced platelet aggregation were the same, which is likely due to simultaneous taking ASA in a low dose.
Omeprazole does not affect the metabolism of drugs metabolized by the CYP3A4 isoenzyme, such as cyclosporine, lidocaine, quinidine, estradiol, erythromycin and budesonide.
No interaction of omeprazole with the following drugs has been identified: antacids, caffeine, theophylline, S-warfarin, piroxicam, diclofenac, naproxen, metoprolol, propranolol and ethanol.
With the simultaneous use of omeprazole and tacrolimus, an increase in the concentration of tacrolimus in the blood serum was noted.
Some patients experienced a slight increase in methotrexate concentrations when combined with proton pump inhibitors. If high doses of methotrexate are prescribed, temporary discontinuation of omeprazole should be considered.
Effect of drugs on the pharmacokinetics of omeprazole
The isoenzymes CYP2C19 and CYP3A4 are involved in the metabolism of omeprazole. The combined use of omeprazole and inhibitors of the CYP2C19 and CYP3A4 isoenzymes, such as clarithromycin and voriconazole, may lead to increased plasma concentrations of omeprazole by slowing down the metabolism of omeprazole. Concomitant use of voriconazole and omeprazole results in a more than twofold increase in the AUC of omeprazole. Due to the good tolerance of high doses of omeprazole, short-term joint use of these drugs does not require dose adjustment of omeprazole.
Co-administration of omeprazole with amoxicillin or metronidazole does not affect the concentration of omeprazole in the blood plasma.
Drugs that induce CYP2C19 and CYP3A4 isoenzymes. such as rifampicin and St. John's wort preparations, when used together with omeprazole, can lead to a decrease in the concentration of omeprazole in the blood plasma by accelerating the metabolism of omeprazole.
Losek Maps price, where to buy
The price of tablets depends on the form of release. The cost of 10 mg (14 pieces in a package) is about 200 rubles. And the price of Losek Maps 20 mg is approximately 230 rubles.
- Online pharmacies in RussiaRussia
ZdravCity
- Losek Maps tablets p.p.o.
20 mg 28 pcs. AstraZeneca 425 rub. order - Losek Maps tablets p.p.o. 20 mg 14 pcs AstraZeneca AB
RUB 227 order
Losek Maps tab p/pl/o 20 mg N14 (AstraZeneca)
Inside, in the morning, the tablet should be swallowed whole, without chewing, with liquid (the tablet can be dissolved in water or a slightly acidified liquid, such as fruit juice; the resulting solution should be used within 30 minutes). Duodenal ulcer: in the acute phase - 20 mg 1 time per day daily for 2 weeks; if there is no effect within 2 weeks, a repeat 2-week course is prescribed. For duodenal ulcer resistant to therapy - 40 mg once a day for 4 weeks. To prevent relapses of duodenal ulcer - 10 mg (if necessary - up to 20-40 mg/day) 1 time per day. Gastric ulcer: 20 mg 1 time per day daily for 4 weeks; if there is no effect, repeat the 4-week course. For gastric ulcers resistant to therapy - 40 mg once a day for 8 weeks. To prevent relapses of gastric ulcers - 20 mg (if necessary - up to 40 mg) 1 time per day. NSAID-associated gastric ulcers or duodenal erosions: 20 mg 1 time per day for 4 weeks. If there is no effect, a repeated 4-week course of treatment is prescribed. Helicobacter pylori eradication regimens for gastric ulcers. Three-component treatment regimen: Losec MAPS 20 mg, amoxicillin 1 g and clarithromycin 500 mg (all drugs taken 2 times a day for 1 week) or Losec MAPS 20 mg, metronidazole 400 mg (or tinidazole 500 mg) and clarithromycin 250 mg (all drugs taken 2 times a day for 1 week), or Losec MAPS 40 mg once a day, amoxicillin 500 mg and metronidazole 400 mg 3 once a day for 1 week. Two-component treatment regimen: Losec MAPS 40–80 mg and amoxicillin 1.5 g daily (the dose should be divided into parts) for 2 weeks. During clinical trials, Losec MAPS 40 mg was used once a day, amoxicillin in a daily dose of 1.5–3 g and clarithromycin 500 mg 3 times a day for 2 weeks. If after treatment the test for Helicobacter pylori remains positive, the course can be repeated. Reflux esophagitis: 20 mg once a day for 4 weeks. If there is no effect, repeat the 4-week course. For patients with severe reflux esophagitis - 40 mg once a day for 8 weeks. For reflux esophagitis in remission - 10 mg once a day (if necessary - up to 20–40 mg/day) for a long time. Symptomatic gastroesophageal reflux disease, dyspepsia associated with increased production of hydrochloric acid: 10–20 mg/day daily for 4 weeks. If symptoms do not disappear at the end of therapy, additional examination of the patient is recommended. Zollinger-Ellison syndrome: the dosage regimen is determined individually. The recommended starting dose is 60 mg/day. In all patients with severe disease, as well as in cases where other therapeutic methods did not lead to the desired result, the drug was effective in more than 90% of patients when taking 20-120 mg of Losec MAPS daily. If the daily dose exceeds 80 mg, it should be divided into 2 hours and taken 2 times a day. In elderly patients, as well as in cases of impaired renal function, dose adjustment is not required. If liver function is impaired, the bioavailability of omeprazole and T1/2 from plasma increases , therefore a dose of 10–20 mg is sufficient.
Losek maps film-coated tablets 20 mg No. 28
Directions for use and doses
Inside.
Losec MAPS tablets are recommended to be taken in the morning; the tablet should be swallowed whole with liquid. Tablets should not be chewed or crushed. The tablets can be dissolved in water or a slightly acidified liquid, such as fruit juice. The resulting solution must be used within 30 minutes. To ensure you take the full dose, fill the glass halfway with liquid again, shake and drink. Duodenal ulcer. Patients with an active duodenal ulcer are recommended to take Losec MAPS 20 mg once daily. The drug provides rapid relief of symptoms. In most patients, ulcer healing occurs within 2 weeks. In cases where complete healing of the ulcer does not occur within 2 weeks, healing is achieved with a subsequent 2-week intake of Losec MAPS.
Patients with duodenal ulcers that are poorly responsive to treatment are usually prescribed Losec MAPS 40 mg once a day; Ulcer healing usually occurs within 4 weeks. To prevent relapses, Losec MAPS 10 mg 1 time per day is recommended for patients with duodenal ulcers. If necessary, the dose can be increased to 20-40 mg 1 time per day. Stomach ulcer.
The recommended dose is Losec MAPS 20 mg once a day. The drug provides rapid relief of symptoms. For most patients, cure occurs within 4 weeks. In cases where complete healing does not occur after the first course of taking the drug, a repeated 4-week course of treatment is usually prescribed, during which healing is achieved. Patients with gastric ulcers that are poorly responsive to treatment are usually prescribed Losec MAPS 40 mg 1 time per day; healing is usually achieved within 8 weeks. To prevent relapses, Losec MAPS 20 mg is recommended to patients with gastric ulcers 1 time per day. If necessary, the dose can be increased to 40 mg 1 time per day. NSAID-associated ulcers and erosions of the stomach and duodenum In the presence of NSAID-associated ulcers of the stomach, duodenum or gastroduodenal erosions in patients with discontinued or ongoing NSAID therapy, the recommended dose of Losec MAPS is 20 mg 1 time per day.
The drug provides rapid relief of symptoms; in most patients, cure occurs within 4 weeks. In those patients who do not heal during the initial therapy period, healing is usually achieved with a repeat 4-week dose of the drug. For the prevention of ulcers and erosions of the stomach and duodenum and symptoms of dyspepsia associated with taking NSAIDs, the recommended dose of Losec MAPS is 20 mg once a day. Helicobacter pylori (Hp) eradication regimens for peptic ulcer disease.
Three-component treatment regimen: Losec MAPS 20 mg, amoxicillin 1 g and clarithromycin 500 mg. Take all drugs 2 times a day for one week or Losec MAPS 20 mg, metronidazole 400 mg (or tinidazole 500 mg) and clarithromycin 250 mg.
Take all drugs 2 times a day for one week or Losec MAPS 40 mg 1 time a day, as well as amoxicillin 500 mg and metronidazole 400 mg 3 times a day for one week. Two-component treatment regimen: Losec MAPS 40-80 mg daily and amoxicillin 1.5 g daily (dose should be divided into parts) for two weeks. During clinical trials, amoxicillin was used at a daily dose of 1.5-3 g, Losec MAPS 40 mg once a day and clarithromycin 500 mg 3 times a day for two weeks. To ensure complete healing, further treatment should be carried out in accordance with the recommendations in the sections “Duodenal Ulcer” and “Gastric Ulcer”. In cases where, after completing a course of treatment, the test for Helicobacter pylori remains positive, the course of treatment can be repeated. Reflux esophagitis.
The recommended dose is one Losec MAPS 20 mg tablet once a day. The drug provides rapid relief of symptoms. For most patients, cure occurs within 4 weeks. In cases where, after the first course of taking the drug, complete cure does not occur, a repeated 4-week course of treatment is usually prescribed, during which a cure is achieved. For patients with severe reflux esophagitis, Losec MAPS 40 mg is recommended once a day; cure usually occurs within 8 weeks. Patients with reflux esophagitis in remission are prescribed Losec MAPS 10 mg once a day as long-term courses of maintenance therapy. If necessary, the dose can be increased to 20-40 mg. Symptomatic gastroesophageal reflux disease. The recommended dose is Losec MAPS 20 mg once a day. The drug provides rapid relief of symptoms. The therapeutic effect can be achieved with a daily dose of 10 mg, so individual dose selection cannot be ruled out. If after 4 weeks of treatment (Losec MAPS 20 mg 1 time per day) symptoms do not disappear, additional examination of the patient is recommended. Dyspepsia associated with high acidity. To relieve pain and/or eliminate discomfort in the epigastric region, with or without heartburn, Losec MAPS 20 mg is prescribed once a day. The therapeutic effect can be achieved with a dose of 10 mg 1 time per day, so treatment can be started with this dose. If after 4 weeks of treatment (Losec MAPS 20 mg 1 time per day) symptoms do not disappear, additional examination of the patient is recommended. Zollinger-Ellison syndrome. For patients with Zollinger-Ellison syndrome, the drug is prescribed in an individual dosage. Treatment is continued according to clinical indications for as long as necessary. The recommended starting dose is Losec MAPS 60 mg daily.
In all patients with severe disease, as well as in cases where other therapeutic methods did not lead to the desired result, the drug was effective in more than 90% of patients when taking 20-120 mg of Losec MAPS daily. In cases where the daily dose of the drug exceeds 80 mg, the dose should be divided into two parts and taken 2 times a day.
Renal dysfunction. For patients with impaired renal function, no dose adjustment is required. Liver dysfunction. In patients with impaired liver function, the bioavailability and plasma half-life of omeprazole are increased. In this regard, a dose of 10-20 mg per day is sufficient. Elderly patients. No dose adjustment is required for elderly patients. Experience in children is limited.