Compound
One bottle of Puregon contains 50 or 100 IU of the active component follitropin beta .
One bottle of Puregon 150 contains 150 IU of follitropin beta .
One bottle of Puregon 300 IU contains 300 IU of follitropin beta .
One bottle of Puregon 600 IU contains 600 IU of follitropin beta .
One bottle of Puregon 900 contains 900 IU of follitropin beta .
The additional ingredients in the medicine are: sodium citrate dihydrate, sucrose, polysorbate 20, L-methionine, benzyl alcohol, hydrochloric acid 0.1 N or sodium hydroxide 0.1 N, water.
How to correctly set the dosage in pens?
Let's talk in more detail about pen syringes: how to use them correctly and how to correctly set the dose of the injected drug. This issue is very important, because incorrect administration of the drug dose can lead to a number of complications, including ovulation hyperstimulation syndrome, which is difficult to treat.
Before you start administering Puregon using a pen syringe, you should prepare a cartridge, pen, sterile disposable needle, alcohol for processing and cotton wool. The optimal place to store open cartridges is the refrigerator, from which you should remove the drug the day before. It can be stored in a room in a dark place for 90 days. A cartridge containing 300 IU of the drug will have a silver cap, a gold cap will contain 600 IU of the drug, and a cartridge with a blue cap will have 900 IU. Only the attending physician selects the dosage of the drug to stimulate ovulation. Before injection, wash your hands with soap twice, prepare the pen and remove the protective cap from it. Carefully remove the body from the pen, open the cartridge, treat it with a disinfectant and place it in a metal holder, then tighten it so that the yellow arrow on the body coincides with the black line on the base of the pen. This will indicate that the cartridge is inserted correctly. After making sure that the cartridge is filled correctly, put a sterile disposable needle on it, from which the protective film is removed and the needle is fixed by screwing it onto the base of the cartridge. After this, remove the cap from the needle and treat the skin. It is better to inject puregon subcutaneously into the abdomen in the fold under the navel or thigh, changing the location each time. After treating the injection site with a disinfectant, you need to let it dry and proceed with the injection itself. First of all, remove the cap from the needle, tap the cartridge to remove air bubbles, turn the handle until it clicks and press the button to inject and administer the drug, as a result of which a drop of liquid should appear.
Setting the dosage
After the syringe is filled, you need to select the dose of the drug. We will tell you how to do this now. So, turn the knob until your prescribed dose appears in the window. If you accidentally scrolled through the dose prescribed for you, then turn the handle all the way, and then press the handle, without losing a single drop. Having established the dose of the drug prescribed to you by your doctor, treat the skin and insert the needle into the subcutaneous fold. By pressing the handle, inject the marked dose, after which for several seconds without removing the syringe from the skin and making sure that the window on the syringe shows 0. After the injection, remove the needle from, close the handle and place it out of reach of children. You can use the started cartridge as long as there is enough drug for one injection. You will use a disposable needle every day and set the appropriate dose of the drug injected.
pharmachologic effect
The active substance that is part of the drug Puregon has a follicle-stimulating effect on the body. Under the influence of the drug, the deficiency of FSH is replenished, the process of normal growth and maturation of follicles is regulated, as well as the synthesis of sex steroid hormones in the body.
Follitropin beta is a recombinant follicle-stimulating hormone that is produced using genetic engineering.
In the female body, the content of follicle-stimulating hormone determines the process of the beginning and duration of maturation of follicles in the ovaries. This hormone also regulates the number of follicles and the maturation period.
The use of Puregon is advisable for the purpose of inducing the development of follicles and steroid hormones in patients diagnosed with ovarian dysfunction. The medicine also stimulates the development and growth of follicles in those women who are planning artificial insemination, in particular, IVF, embryo transfer, gamete transfer into the fallopian tubes, or intracytoplasmic sperm injection.
After a course of Puregon therapy has been completed, it is recommended to administer human chorionic gonadotropin to induce the last stage of the follicle maturation process.
Puregon is also used by men to treat follicle-stimulating hormone deficiency, leading to a decrease in spermatogenesis. In this case, the medicine is used in combination with human chorionic gonadotropic hormone, and therapy should last at least 4 months.
Puregon solution for subcutaneous administration 300ME, 1 cartridge
Registration Certificate Holder
NV ORGANON (Netherlands)
Dosage form
Medicine - Puregon® (Puregon)
Description
Solution for subcutaneous administration
transparent, colorless.
1 cartridge
follitropin beta (recombinant)* 300 IU
Excipients
: sucrose - 21 mg, sodium citrate dihydrate - 6.17 mg, polysorbate 20 - 0.105 mg, benzyl alcohol - 4.2 mg, methionine - 0.21 mg, hydrochloric acid 0.1 N or sodium hydroxide 0.1 N - up to pH 7, water d/i - 0.42 ml.
0.36 ml - 1.5 ml colorless glass cartridges (1) complete with needles (6 pcs.) - plastic packaging (1) - cardboard packs.
* - specific biological activity in vivo is approximately 10,000 IU FSH/ml protein.
Indications
Infertility due to anovulation (including polycystic ovary syndrome); induction of superovulation in the following infertility treatment programs: in vitro fertilization/embryo transfer, gamete transfer into the fallopian tube, intracytoplasmic sperm injection.
Contraindications for use
Tumors of the ovaries, mammary glands, uterus, pituitary gland or hypothalamus; ovarian wasting syndrome; ovarian cysts (but not polycystic ovary syndrome); developmental abnormalities of the genital organs and/or benign tumors (myomas and fibroids) of the uterus that are incompatible with pregnancy; extragenital endocrinopathies caused by tumors of the thyroid gland, adrenal glands, pituitary gland; uterine bleeding of unknown etiology; pregnancy; lactation; hypersensitivity to follitropin beta.
pharmachologic effect
Recombinant FSH. It has a more pronounced effect than FSH obtained from the urine of postmenopausal women.
Dosage regimen
Initial daily doses are 50-100 IU for induction of ovulation (with anovulatory infertility) and 100-250 IU for induction of superovulation (in assisted reproduction programs). Further dosing regimen depends on the response of the ovaries to stimulation of ovulation, which is monitored using ultrasound, as well as measuring the level of estradiol in the blood. The final stage of ovulation stimulation (with anovulatory infertility) or preparation of mature follicles for puncture (in assisted reproduction programs) is carried out by intramuscular injection of 5-10 thousand IU of hCG. The criterion for follicle maturity is its diameter of 18 mm or more.
Side effect
Ectopic pregnancy, multiple pregnancy. It is possible to develop ovarian hyperstimulation syndrome, which is manifested by the following symptoms: mild form - pain in the lower abdomen, nausea, diarrhea, slight enlargement of the ovaries, the appearance of ovarian cysts; severe form - the appearance of large ovarian cysts (prone to rupture), ascites, hydrothorax; weight gain and, in rare cases, thromboembolism.
Mild hyperemia at the injection site is possible.
special instructions
It should be kept in mind that follitropin beta is more potent than FSH extracted from postmenopausal urine, so a lower dose of follitropin beta is required per stimulation cycle.
When using follitropin beta to stimulate ovulation, early and constant monitoring with ultrasound is necessary, because there may be an increased risk of ectopic or multiple pregnancies and ovarian hyperstimulation syndrome.
Pre-treatment with clomiphene may enhance the stimulation of ovulation by follitropin beta. After use of GnRH agonists, higher doses of follitropin beta may be required for treatment.
Pharmacokinetics and pharmacodynamics
If Puregon is administered subcutaneously, the highest concentration of the active substance in plasma is observed after 12 hours. Since the absorption process of the active component is slow, and its half-life is 12-70 hours, there is a high level of follicle-stimulating hormone in the body for 24-48 hours after the injection. If the same dose of the drug is repeated, a further increase in follicle-stimulating hormone is noted: its level increases by 1.5-2 times when compared with the first injection. Therapeutic doses of follicle-stimulating hormone in plasma are observed after repeated administration. The bioavailability level is 77%.
There are practically no differences in the pharmacokinetic profile when the solution is administered intramuscularly and subcutaneously. The biochemical similarity of the follicle-stimulating hormone in Puregon with the hormone obtained from human urine is noted; it has a similar metabolic profile, is distributed and excreted from the body in a similar way.
Indications for use
The medicine Puregon is prescribed to women in the following cases:
- for female infertility due to anovulation (including polycystic ovary syndrome , treatment of which with clomiphene citrate is ineffective);
- for the purpose of conducting assisted reproduction programs , including IVF, embryo transfer, sperm injections (for the purpose of inducing superovulation).
The drug is also prescribed for the treatment of men who have insufficient spermatogenesis associated with hypogonadotropic hypogonadism .
Contraindications
Puregon should not be used to treat those patients who have an intolerance to the components of this drug, as well as neomycin or streptomycin (these components may be present in the solution).
The drug is not used to treat people diagnosed with hormone-dependent tumors ( tumors of the breast , ovaries or testicles , pituitary gland , uterus , hypothalamus ).
Not used to treat patients diagnosed with primary gonadal failure .
Puregon should not be prescribed to women with disorders of the anatomy of the genital organs, as well as vaginal bleeding of unknown origin.
It is not prescribed to patients with uterine fibroids, which are incompatible with pregnancy.
The drug should not be prescribed for the treatment of women suffering from ovarian cysts , as well as to patients with enlarged ovaries not associated with polycystic ovary syndrome .
Before you start using Puregon, you need to exclude diseases of the endocrine system that are not associated with dysfunction of the gonads.
Prescribe the medicine with caution to women who have undergone abdominal surgery. In patients with polycystic ovary syndrome and ovarian cysts, treatment with Puregon increases the likelihood of ovarian torsion due to hyperstimulation. Therefore, in this case, constant monitoring of the anatomical position of the ovary is necessary.
Prescribe the medicine with caution to women who have an increased risk of thrombosis , as they are more likely to develop thromboembolism .
Puregon®
Puregon® may contain traces of streptomycin and/or neomycin. These antibiotics may cause hypersensitivity reactions.
— Before starting treatment, a married couple with infertility should be properly examined. Namely, hypothyroidism, adrenal insufficiency, hyperprolactinemia, tumors of the pituitary gland or hypothalamus should be excluded. If necessary, treat these diseases.
Among women
— OHSS is an iatrogenic condition, which is based on the response of the ovaries to the exogenous administration of ovulation inducing drugs, exceeding the physiological limits. Clinical manifestations and symptoms of mild to moderate OHSS: abdominal pain, nausea, diarrhea, mild/moderate enlargement of the ovaries, ovarian cysts. Clinical manifestations and symptoms of severe OHSS: large ovarian cysts, acute abdominal pain, ascites, pleural exudate, hydrothorax, dyspnea, oliguria, hematological disorders, weight gain. Severe OHSS may be complicated by venous and arterial thrombosis and thromboembolism. Against the background of OHSS, there have been cases of transient disturbances in liver function tests, indicating organ dysfunction, both in combination with morphological changes according to biopsy data, and without them.
OHSS can be caused by the use of hCG and pregnancy (endogenous hCG). Typically, early manifestations of OHSS are observed within 10 days after the use of hCG. These phenomena are associated with an excessively pronounced ovarian response to gonadotropin stimulation. Late manifestations of OHSS occur more than 10 days after the use of hCG and occur as a result of changes in hormonal balance during pregnancy. Given the risk of developing OHSS for at least 2 weeks after hCG administration, monitoring is required.
Women with known risk factors for increased ovarian response are especially prone to developing OHSS during or after use of Puregon. During the first cycle of ovarian stimulation, when risk factors are only partially known, careful monitoring of early symptoms of OHSS is required.
In order to reduce the risk of developing OHSS, it is advisable to conduct an ultrasound to assess the size of the follicles before starting a course of therapy and then regularly throughout the course of therapy. Parallel determination of serum estradiol concentration is also necessary.
Assisted reproductive technologies (ART) are characterized by an increased risk of OHSS in the presence of 18 or more follicles with a diameter of 11 mm or more. If you have 30 follicles or more, it is recommended to refrain from using hCG.
Measures to reduce the risk of developing OHSS depending on the severity of the ovarian response
- Termination of further stimulation with gonadotropin for a maximum period of 3 days.
— Cancellation of hCG and termination of the therapeutic cycle.
- To activate the final maturation of the egg, the use of hCG (human chorionic gonadotropin isolated from urine) in a dose of less than 10,000 IU, for example, 5000 IU of hCG isolated from urine, or 250 mcg of chorionic gonadotropin alpha obtained by recombinant technology, which is equivalent to approximately 6500 IU of hCG isolated from urine.
Cancellation of embryo transfer followed by cryopreservation.
Cancellation of hCG to support the luteal phase.
In case of development of OHSS, standard therapeutic measures are recommended.
Cases of ovarian torsion have been reported following therapy with gonadotropins, including Puregon®. Ovarian torsion may be associated with other risk factors, such as OHSS, pregnancy, history of abdominal surgery and ovarian torsion, current or history of ovarian cysts/polycystic disease.
Damage to the ovaries due to decreased blood supply can be minimized with early diagnosis and immediate medical intervention.
Thromboembolic events, both associated and unrelated to OHSS, have been reported following the use of gonadotropins, including Puregon. Vascular thrombosis, both venous and arterial, can lead to a decrease in blood supply to vital organs or limbs. In women with known risk factors for thromboembolic events (personal or family history, severe obesity, thrombophilia), the use of gonadotropins, including Puregon®, may further increase the risk of developing OHSS. In such cases, the risks and benefits of using gonadotropins, including Puregon, should be carefully assessed. It should be noted that pregnancy also increases the risk of thrombosis.
— With the use of gonadotropins, including the drug Puregon®, cases of multiple pregnancies with subsequent births have been reported. In many cases, with multiple pregnancies, there was an increased risk of adverse events for the mother (complications of pregnancy and childbirth), as well as for the newborn (low birth weight). To minimize the risk of multiple pregnancy in patients with anovulation during ovulation induction, it is advisable to conduct transvaginal ultrasound monitoring of follicle development. It is also advisable to determine the concentration of estradiol in the blood serum. Patients should be informed of the risk of developing multiple pregnancies before initiating therapy.
During ART, the risk of multiple pregnancy is mainly associated with the number of embryos transferred. When inducing ovulation, adjusting the dose of FSH prevents multiple growth of follicles.
Women undergoing ART procedures often have fallopian tube abnormalities, which increases the risk of developing an ectopic pregnancy. For such patients, it is important to conduct an early ultrasound examination to confirm the intrauterine location of the ovum.
The incidence of congenital malformations with ART may be slightly higher than with natural fertilization. This may be due to parental characteristics, such as maternal age or paternal sperm characteristics, as well as a higher incidence of multiple pregnancies with ART. There has been no evidence that an increased risk of congenital defects is associated with the use of gonadotropins.
— Cases of the development of neoplasms of the ovaries and other organs of the reproductive system, both benign and malignant, have been reported in women who underwent various types of therapy for infertility. At the moment, no relationship has been established between the use of gonadotropins in the treatment of infertility and an increased risk of developing tumors in women.
— Before starting to use Puregon, medical conditions that would contraindicate pregnancy should be excluded.
In men
— Increased concentrations of endogenous FSH in men indicate primary testicular failure. In such patients, combination therapy with Puregon and hCG is ineffective
Side effects
When treated with Puregon, some patients may develop a number of local reactions in the place where the solution was injected. This may be hyperemia, pain, swelling, or the appearance of a rash.
Systemic allergic manifestations were rarely recorded during treatment.
The use of follitropin beta in women can provoke the manifestation of ovarian hyperstimulation with pain and congestion in the pelvic area, headache, bloating , pain in the abdominal and epigastric region. Also, with this phenomenon, a woman may experience an increase in the size of her ovaries and experience chest pain. Possible metrorrhagia , development of ovarian torsion, ovarian cysts , and bleeding from the vagina.
There have been isolated cases of severe ovarian hyperstimulation syndrome , a life-threatening condition. In this condition, a woman may develop large cysts, which leads to the risk of their rupture, ascites , and weight gain due to fluid retention in the body. If such a side effect occurs, you should immediately stop treatment and consult a specialist.
ectopic pregnancy , multiple pregnancy , and miscarriage during the use of the drug .
When using combined treatment with hCG and Puregon, there is a possibility of developing thromboembolism .
If the medicine is used by men, they may experience headache as a side effect. It is also possible to develop acne , epididymal cysts , gynecomastia , and allergic manifestations.
Puregon, instructions for use (Method and dosage)
Puregon is administered parenterally - intramuscularly or subcutaneously.
The solution cannot be injected into the vascular cavity, therefore, before injecting the product, it is necessary to exclude the possibility of it getting into the vessel. Treatment with the drug is carried out under the supervision of a doctor who has experience in the treatment of human reproductive disorders. It is important to monitor the patient's condition when the solution is first administered.
The solution can be administered using a disposable syringe or a special injector pen. If a syringe is used for administration, it is important to note that in this case, the patient is injected with 18% less follicle-stimulating hormone compared to using a cartridge using a Puregon Pen pen. The video instructions for Puregon Pen will help you make this introduction correctly.
It is recommended to administer the solution subcutaneously slowly to avoid pain and leakage of the solution. With repeated injections, it is important to change the injection site to prevent atrophy of the adipose tissue. The patient can self-administer the medication, but only after being instructed by a healthcare professional. Before administering the drug, you need to check whether there are any foreign particles in it and whether the transparency is broken - in such cases the medicine cannot be administered.
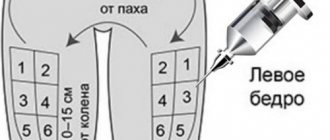
Once the bottle has been opened, the solution cannot be stored. To achieve the greatest effect, it is recommended to inject Puregon into the stomach - in the navel area. Before injecting the product, it is important to wash your hands and treat the area where the product will be injected with a disinfectant solution.
To introduce the product, you need to pull back the skin, form a fold and insert a needle into it perpendicular to the skin. In this case, you need to make sure that the needle does not get into the vessel. After the drug has been administered, it is necessary to lightly massage the skin at the site of injection of the solution.
The dosage of the drug and the duration of its administration are determined by a specialist, taking into account the reaction of the ovaries in women. During therapy, it is imperative to perform an ultrasound of the ovaries and determine the content of estradiol in plasma. Clinical experience shows that it is possible to achieve an effect after three or more courses of medication.
As evidenced by the experience of using Puregon in women before artificial insemination, it is most likely to be successful during the first four courses. Further efficiency decreases.
Women diagnosed with anovulation are advised to practice a consistent regimen of medication. In the first week of treatment, 50 IU of Puregon should be administered per day. If there is no ovarian response, the daily dose should be gradually increased until estradiol levels or follicular growth are sufficient. The optimal treatment pattern is an increase in plasma estradiol concentrations by 40-100%. preovulation occurs . This state is usually achieved after 1-2 weeks of Puregon administration. Next, the administration of the Puregon solution should be stopped and the administration of hCG should be started to induce ovulation. The dose of Puregon is reduced if a large number of follicles respond to therapy or if there is an increase in estradiol levels by more than 2 times for two or three days in a row. If several follicles that are larger than 14 mm develop, there is a possibility of multiple pregnancy . HCG should not be administered if multiple follicles are developing. In this case, measures should be taken to prevent the development of multiple pregnancies.
When inducing hyperovulation, different treatment regimens are used. First, the drug is administered at a dose of 100–225 IU for at least 4 days. Next, the doctor sets an individual dosage, taking into account the response of the ovaries to treatment. As a rule, it is sufficient to administer a maintenance dose of 75–375 IU for 6–12 days. Sometimes longer treatment is practiced.
Puregon is used both as a drug for monotherapy and in combination with an agonist or antagonist of gonadotropin-releasing hormone to prevent the premature formation of the corpus luteum. Sometimes this combination requires the use of higher doses of Puregon.
Ultrasound should be performed to monitor the ovarian response and determine plasma estradiol concentrations. If the presence of at least three follicles with a diameter of 16–20 mm is noted, and there is also evidence of a good ovarian response, hCG is administered to indicate the final phase of follicle maturation. After 34–35 hours, oocyte aspiration is performed.
For the treatment of men, the drug is used at a dose of 450 IU per week, it should be administered in three doses of 150 IU. Puregon is combined with hCG. As a rule, improvement in spermatogenesis occurs no earlier than after 3–4 months. It is necessary to conduct a semen analysis 4-6 months after the start of treatment to determine how effective the therapy is. If there is no positive effect, treatment is continued. It may take about 18 months to restore spermatogenesis.
Release form and packaging Puregon
The drug is available in the form of a solution that is administered intramuscularly or subcutaneously. Considering that the drug is administered according to the prescribed scheme, for convenience it is manufactured in cartridges that fit into special pens (Puregon Pen), which allows you to make injections yourself without much difficulty. Thanks to this form of release, a woman can administer it herself.
Cartridges come in several options: 0.36 ml, 0.72 and 1.08 ml and come with a set of needles. To reduce pain during an injection, as well as to use up the solution during manipulation, today they use syringes that come with small needles or a special Puregon pen. This is a special device that allows you to avoid leakage of the drug during manipulation by medical workers or self-use, because if the drug is not in full, accordingly, the dose that is required to stimulate ovulation will not be completely administered.
special instructions
Before starting treatment, endocrine diseases .
Before starting therapy, the patient should be warned about the possibility of multiple pregnancy. Correcting the dosage of follitropin beta helps prevent the development of multiple follicles.
Puregon should be administered for the first time only under the supervision of a specialist. Before you administer the solution yourself, you need to consult a doctor and watch a video on how to inject Puregon
Those women who undergo artificial insemination should take into account that they often have abnormalities of the fallopian tubes, which increases the risk of developing an ectopic pregnancy. Therefore, it is necessary to confirm by ultrasound that the fetus is intrauterine. It should also be taken into account that with artificial insemination the risk of early pregnancy termination is higher than in the case of natural conception.
During the treatment process, it is important to regularly perform control ultrasounds.
The product may contain traces of Streptomycin , neomycin .
Women with thrombophilia or obesity have an increased risk of thromboembolism during Puregon therapy.
Puregon 900
Treatment with Puregon® should be started under the supervision of a physician experienced in the treatment of infertility.
The dose should be adjusted individually depending on the response of the ovaries, under ultrasound control and estradiol concentration.
Puregon® is effective with a lower total dose and shorter treatment time required for maturation compared to FSH obtained from urine, which minimizes the risk of developing ovarian hyperstimulation.
Overall experience in the treatment of infertility by in vitro fertilization indicates that success is most likely during the first 4 courses of therapy and gradually decreases thereafter.
During anovulation
A sequential treatment regimen is recommended, starting with daily administration of 50 IU of Puregon® for at least 7 days. In the absence of an ovarian response, the daily dose is gradually increased until follicular growth and/or an increase in plasma estradiol concentration is achieved, indicating that an optimal pharmacodynamic response has been achieved. A daily increase in plasma estradiol concentration by 40-100% is considered optimal.
The daily dose thus obtained is then maintained until the preovulation state is achieved. The state of preovulation is determined by the presence of a dominant follicle with a diameter of at least 18 mm (according to ultrasound) and/or a plasma extradiol concentration of 300-900 picograms/ml (1000-3000 pmol/l).
Typically, 7-14 days of treatment are required to achieve this state.
After this, the administration of the drug is stopped and ovulation is induced by administering hCG. If the number of follicles is too large or the estradiol concentration increases too quickly, i.e. more than 2 times per day for 2-3 consecutive days, then the daily dose should be reduced. Since each follicle with a diameter greater than 14 mm is preovulatory, the presence of several follicles with a diameter greater than 14 mm carries the risk of multiple pregnancies. In this case, hCG is not administered and measures are taken to protect against possible pregnancy to prevent multiple pregnancies.
For induction of superovulation during artificial insemination
Various stimulation schemes are used. For at least the first 4 days, it is recommended to administer 150-225 IU of the drug. After this, the dose can be selected individually, based on the reaction of the ovaries. Clinical studies have shown that a maintenance dose of 75-375 IU for 6-12 days is usually sufficient, but in some cases longer treatment may be required.
Puregon® can be used either alone or in combination with a GnRH agonist or antagonist to prevent premature peak ovulation. When using GnRH analogues, higher total doses of Puregon® may be required.
The reaction of the ovaries is monitored by ultrasound and determination of the concentration of estradiol in plasma. If there are at least 3 follicles with a diameter of 16-20 mm (according to ultrasound) and there is a good ovarian response (estradiol concentration in blood plasma 300-400 picograms/ml (1000-1300 pmol/l) for each follicle with a diameter of more than 18 mm), induce the final phase of follicle maturation by administering hCG. After 34-35 hours, oocytes are aspirated.
Rules for using the drug
To prevent pain during injection and to minimize leakage of the drug from the injection site, the solution should be administered slowly IM and SC. It is necessary to alternate sites of subcutaneous injection to avoid the development of fatty atrophy. Unused solution should be destroyed.
Subcutaneous injections of Puregon® can be carried out by the woman herself or her partner, who has received detailed instructions from the doctor. Self-administration of the drug is permissible only for patients who have good skills and a constant opportunity to consult with a specialist.
The drug is produced in cartridges
, intended for administration using
the Puregon Pen injector pen.
In this case, the drug is administered subcutaneously.
When using the Puregon Pen injector pen, it must be taken into account that the pen is a precise device that releases the dose set on it. It has been shown that using an injector pen delivers 18% more FSH than using a syringe. This may be significant, in particular, when changing an injector pen to a regular syringe, and vice versa, in the same treatment cycle. Some dose adjustment is especially necessary when switching from a syringe to a pen to avoid an unacceptable increase in the administered dose.
The drug is available in bottles
, intended for administration using
a syringe
.
Step 1 - Preparing the syringe
To administer the drug, disposable sterile syringes and needles should be used. The volume of the syringe must be small enough to deliver the prescribed dose accurately. If the solution is opaque or contains mechanical inclusions, it cannot be used. The contents of the bottle should be used immediately after piercing the rubber stopper. The solution remaining after a single use is discarded. First, remove the valve from the bottle cap. Place the needle on the syringe and pierce the rubber stopper of the bottle with the needle. Draw the solution into the syringe and replace the needle with an injection needle. Holding the syringe with the needle up, gently tap it on the side to displace air bubbles into the upper part of the syringe, then press the piston until the air is completely removed, until only the Puregon® solution remains in the syringe. If necessary, additional pressure on the piston is used to set the volume of the solution intended for administration.
Stage 2 - Injection site
The most suitable place for subcutaneous injection is the abdominal area around the navel with mobile skin and a layer of fatty tissue. With each injection, you should change the injection site slightly. The drug can be injected into other areas of the body.
Stage 3 - Preparing the injection site
To reduce discomfort when inserting a needle, you can make several claps at the site of the intended injection. Hands should be washed and the injection site should be wiped with a disinfectant solution (for example, 0.5% chlorhexidine) to remove surface bacteria. Apply approximately 6 cm around the point where the needle will enter and wait about a minute for the disinfectant solution to dry.
Step 4 - Inserting the needle
Pull the skin back a little. With your other hand, insert the needle at a 90° angle under the surface of the skin.
Step 5 - Checking the correct needle position
When the needle is positioned correctly, the piston is quite difficult to return.
Blood seeping into the syringe indicates that the needle has pierced a vein or artery. In this case, remove the syringe, cover the injection site with a swab containing disinfectant liquid and apply pressure, and the bleeding will stop in 1-2 minutes. Do not use the solution and remove it from the syringe. Start again from step 1, using a new needle and syringe, and a new bottle of the drug.
Stage 6 - Introduction of the solution
Lower the plunger slowly and gradually to ensure proper injection of the solution without damaging the skin tissue.
Step 7 -
Removing the syringe
Quickly remove the syringe, cover the injection site with a swab of disinfectant liquid and apply pressure. Gently massaging this area (with constant pressure) will help distribute the Puregon® solution and help avoid discomfort.
Analogs
Level 4 ATX code matches:
Pergoveris
Menopur
Horagon
Ovitrel
Chorionic gonadotropin
Decayed
Gonal-F
There are a number of medications whose active substance is follitropin beta . These are Metrodin , Gonal-F , FSH-Super , Follitropin , Urofollitropin .
Luveris lyophilisate , Ovitrel lyophilisate , Menopur lyophilisate , HuMoG lyophilisate , etc. have a similar effect on the body
Which is better: Gonal or Puregon?
The active component of the drug Gonal is follitropin alfa. This remedy also has a follicle-stimulating effect. There are a number of positive reviews from women who have been treated with this fertility drug. But only a doctor should decide on the choice of medicine.
Reviews about Puregon
Women who leave reviews about Puregon on forums often write about this medicine in a positive way. Those who have been helped by the drug note that it is generally well tolerated and is also easy and convenient to use. Many women write that the medicine effectively stimulates the growth of follicles and helps to get pregnant.
Negative opinions are less common, but patients often note as a negative that the drug is expensive. Reviews and treatment results described by women can be read on thematic websites.