Creon 25000 300 mg No. 20 caps. with mk/spheres.
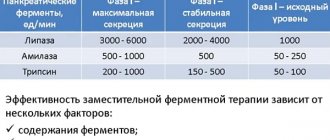
Trade name Creon® 25000 International nonproprietary name No Dosage form Capsules containing enteric-coated minimicrospheres, 300 mg Composition One capsule contains the active substance - pancreatin 300 mg, with minimal enzymatic activity: amylase - 18,000 Euro units. F., lipases - 25,000 EUR units. F., proteases - 1000 EUR units. F., produced from pancreatic tissue of porcine origin, excipients pellet core: macrogol 4000, pellet shell: hypromellose phthalate, cetyl alcohol, triethyl citrate, dimethicone 1000, capsule shell: gelatin, anhydrous iron oxide III (E 172), hydrated iron oxide III (E 172), titanium dioxide (E 171), sodium lauryl sulfate. Description Hard, gelatin capsules, size No. 0, with a red-brown cap and a colorless body, filled with brown mini-microspheres (pellets). Pharmacotherapeutic group Drugs that promote digestion (including enzyme preparations). Digestive enzyme preparations. Pancreatin Code ATC A09AA02 Pharmacological properties Pharmacokinetics It is known that intact enzymes are not absorbed, therefore classical studies on the pharmacokinetics of Creon® 25000 have not been conducted. Pancreatic enzymes do not require absorption to function. On the contrary, the full therapeutic effect occurs in the lumen of the gastrointestinal tract. Because they are protein molecules, enzymes undergo further proteolytic digestion as they move through the gastrointestinal tract until they are absorbed as peptides or amino acids. Pharmacodynamics Creon® 25000 capsules contain pancreatin of porcine origin in the form of minimicrospheres coated with an enteric (acid-resistant) coating. The capsule shell quickly dissolves in the stomach, releasing hundreds of minimicrospheres. In this case, minimicrospheres are mixed with chyme already in the stomach, which significantly increases the contact area between the food bolus and pancreatic enzymes. When the minimicrospheres reach the small intestine, their enteric coating is rapidly destroyed (at pH > 5.5) with the subsequent release of enzymes with lipolytic, amylolytic and proteolytic activities, resulting in the disintegration of fat, starch and protein molecules. The products of pancreatic digestion then undergo absorption or subsequent hydrolysis by intestinal enzymes. Results of clinical studies A total of 23 clinical studies of the effectiveness of Creon® were conducted in patients with exocrine pancreatic insufficiency. However, 7 of them were placebo-controlled or studies that assessed the effectiveness of treatment relative to the initial condition, involving patients with cystic fibrosis, chronic pancreatitis or after surgical interventions. In all randomized, placebo-controlled trials, the primary endpoint was the superiority of Creon® over placebo in the primary efficacy parameter, fat absorption coefficient (FA). All studies, regardless of disease etiology, showed significant improvements in specific symptoms (such as stool frequency and consistency, flatulence and abdominal pain). Children In cystic fibrosis, the effectiveness of Creon® has been demonstrated in three placebo-controlled studies in children and adolescents with cystic fibrosis, as well as in one baseline-controlled study in infants. A total of 118 patients participated in these studies. The dynamics of LCP shows that there are no differences in the effectiveness of Creon® due to the age of patients. Indications for use For replacement purposes in exocrine pancreatic insufficiency - cystic fibrosis - chronic pancreatitis - condition after pancreatectomy - pancreatic cancer - condition after complete or partial resection of the stomach (gastroenterostomy according to Billroth-II) - obstruction of the pancreatic ducts or common bile duct (in including due to neoplasm) - Shwachman-Diamond syndrome - acute pancreatitis during the period of restoration of enteral nutrition. Method of administration and dosage. Doses of the drug are selected individually depending on the severity of the disease and the composition of the diet. Creon® 25000 capsules are taken orally during meals. If it is necessary to take more than 1 capsule of Creon® 25000, take 1 capsule before and the rest during meals. Capsules should be swallowed whole, without breaking them or chewing them, with a sufficient amount of liquid. If swallowing is difficult (for example, in small children or elderly patients), the capsules are carefully opened, and the minimicrospheres are added to soft food that does not require chewing (for example, applesauce), or taken with liquid. In this case, the food or liquid with which the minimicrospheres are mixed must be acidic (fruit juice or yogurt) to prevent premature release and destruction of enzymes (pH <5.5). Chewing or damage to minimicrospheres may disrupt the protective enteric coating, resulting in premature release of enzymes that may cause irritation of the oral mucosa and/or reduce the therapeutic effect of the drug. Any mixture of minimicrospheres with food or liquid cannot be stored and should be taken immediately after preparation. It is important to constantly take enough fluid, especially if there is increased fluid loss. Not taking in enough fluids can cause constipation. If the patient forgot to take Creon® 25000 on time, you can take the missed dose immediately after a meal. A later appointment is not advisable. At your next meal, you should take the usual dose of the drug. Do not take a double dose to make up for a missed dose. Cystic fibrosis For cystic fibrosis, the dosage of the drug is selected by the attending physician. According to the recommendations of the American Cystic Fibrosis Foundation, the dose of Creon® 25000 is calculated in the number of lipase units per 1 kg of body weight per meal. Depending on age, a single dose is calculated as follows: Age of the child Recommendations Up to 4 years 1000 units. lipase per 1 kg of body weight Over 4 years 500 units. lipase per 1 kg of body weight Dosage and duration of treatment are determined depending on the severity of the disease, control of steatorrhea and maintenance of good nutritional status. In most patients, the dose should not exceed 10,000 lipase units/kg body weight per day or 4,000 lipase units per gram of fat in food. For optimal individual dosing, in addition to Creon® 25000, there is a dosage with a lower enzyme content. Since it is difficult to divide the contents of the Creon® 25000 capsule into several doses, it is recommended to start treatment in children weighing at least 25 kg. For use in children weighing less than 25 kg, a dosage with a lower enzyme content is recommended (Creon® 10000). Dosage for other conditions accompanied by exocrine pancreatic insufficiency. The dosage and duration of treatment should be determined taking into account the individual characteristics of the patient, which include the degree of digestive disturbance and the fat content of food. The dose that the patient requires along with main meals (lunch, breakfast or dinner) can vary from 25,000 to 80,000 units. lipase (Eur. F.), which is from 1 to 3 capsules of Creon® 25000, and when taking a light snack between meals, the dose is approximately half the individual dosage, or 1/2-2 capsules. Side effects Very common - abdominal pain* Common - nausea, vomiting, flatulence, diarrhea, constipation Uncommon - rash Frequency unknown - fibrosing colonopathy, hypersensitivity reactions (anaphylactic reactions), allergic skin reactions: urticaria, itching *Gastrointestinal disorders associated with main disease. The incidence of abdominal pain and diarrhea was similar to or lower than in the placebo group. Fibrosing colonopathy has been described in patients with cystic fibrosis who took high doses of drugs containing pancreatin. In clinical studies involving pediatric patients, no additional adverse reactions were identified. Contraindications - increased individual sensitivity to porcine pancreatin or any other component of the drug. Drug interactions There are no reports of interactions with other drugs or other forms of interaction. When taken simultaneously, the absorption of folic acid decreases, which requires an increase in the dose of the latter, as well as a decrease in the effect of the drugs acarbose and miglitol. Special instructions Strictures of the ileocecal angle and large intestine (fibrosing colonopathy) have been described in patients with cystic fibrosis who took high doses of pancreatin. As a precaution, it is recommended that any unusual symptoms or changes in the gastrointestinal tract be subjected to a thorough medical evaluation to rule out involvement of the colon. Especially if the patient takes more than 10,000 lipase units/kg body weight per day. Like other products containing pancreatins, Creon® 25000 is made from pancreatic tissue from pigs specially raised for human consumption. Although the risk of transmission of infectious agents to humans by Creon® 25000 has been minimized by testing and inactivating certain viruses during the manufacturing process, there is a theoretical risk of transmission of viral disease, including diseases caused by new or unidentified viruses. The presence of swine viruses that could infect humans cannot be completely excluded. However, to date there are no reports of infectious disease transmission associated with the use of porcine pancreatin, while this substance has been used for a long period of time. Pregnancy and lactation Creon® 25000 is prescribed with caution during pregnancy. Due to the lack of systemic absorption of pancreatic enzymes, during breastfeeding Creon® 25000 is prescribed in doses necessary to ensure adequate nutritional status. Features of the effect of the drug on the ability to drive a vehicle or potentially dangerous mechanisms Creon® 25000 does not affect the ability to drive a car or operate machines and mechanisms. Overdose Symptoms: Doses of Creon® 25000, much higher than therapeutic doses, can cause hyperuricosuria and hyperuricemia. Treatment: discontinuation of the drug, sufficient fluid intake, supportive measures. Release form and packaging 20, 50, 100 capsules in white high-density polyethylene bottles, sealed with a screw cap with a tamper evident device. Labels made of self-adhesive paper are glued onto the bottles. Each bottle, along with instructions for medical use in the state and Russian languages, is placed in a box made of cardboard. Storage conditions Store at a temperature not exceeding 25°C in tightly closed packaging. Keep out of the reach of children! Shelf life: 3 years Do not use after expiration date. Do not use after 3 months after opening the bottle. Conditions for dispensing from pharmacies Without a prescription Name and country of the manufacturing organization Abbott Laboratories GmbH, Germany. 31535, Neustadt am Rübenberge, Justus-von Liebig Strasse, 33. Name and country of the owner of the registration certificate Abbott Laboratories GmbH, Germany Name and country of the packing organization Abbott Laboratories GmbH, Germany Address of the organization receiving claims from consumers regarding product quality in the territory of the Republic of Kazakhstan : Representative office in the Republic of Kazakhstan, Dostyk Ave. 117/6, Business, 050059, Almaty, Republic of Kazakhstan. Tel.: +77272447544, fax: +77272447644. e-mail
Kreon® 25000 (Kreon® 25000)
Inside.
Doses of the drug are selected individually depending on the severity of the disease and the composition of the diet.
Capsules should be taken during or immediately after each meal (including snacks), swallowed whole, not broken or chewed, and washed down with sufficient liquid.
If swallowing is difficult (for example, in young children or elderly patients), the capsules are carefully opened and the minimicrospheres are added to soft, non-chewable food that has a sour taste (pH < 5.5), or taken with a liquid that also has a sour taste ( pH < 5.5). For example, minimicrospheres can be added to applesauce, yogurt, or fruit juice (apple, orange, or pineapple) with a pH less than 5.5. It is not recommended to add the contents of the capsules to hot food. Any mixture of minimicrospheres with food or liquid cannot be stored and should be taken immediately after preparation.
Crushing or chewing minimicrospheres, or mixing them with food or liquid with a pH greater than 5.5, can destroy their protective enteric coating. This can lead to early release of enzymes in the mouth, reduced effectiveness and irritation of the mucous membranes. You need to make sure that there are no mini-microspheres left in your mouth.
It is important to ensure that the patient maintains adequate fluid intake, especially if there is increased fluid loss. Inadequate fluid intake may cause or worsen constipation.
Dose for adults and children with cystic fibrosis
- The dose depends on body weight and should be at the beginning of treatment 1000 lipase units/kg at each meal for children under four years of age and 500 lipase units/kg at meals for children over four years of age and adults.
— The dose should be determined depending on the severity of symptoms of the disease, the results of control of steatorrhea and maintenance of adequate nutritional status.
- In most patients, the dose should remain less than or not exceed 10,000 lipase units/kg body weight per day or 4000 lipase units/g fat consumed.
Dose for other conditions accompanied by exocrine pancreatic insufficiency
The dose should be set taking into account the individual characteristics of the patient, which include the degree of digestive insufficiency and the fat content of food. The dose required by the patient with the main meal varies from 25,000 to 80,000 units of lipase, and half the individual dose when taking a snack.
In children, the drug should be used as prescribed by a doctor.