Composition and release form
Ingaron contains both the main component and excipients. Interferon gamma is used as the active ingredient. Additionally, the drug contains mannitol.
The drug externally is a porous, loose, hygroscopic mass. The drug is white in color and is available in the form of a lyophilisate, which is used to prepare nasal drops.
When purchasing a medicine, the kit includes 1 bottle of Ingaron, 1 ampoule with 5 milliliters of solvent and 1 dropper cap or pipette for preparing an intranasal solution. The manufacturer also includes detailed instructions for use in the box.
pharmachologic effect
The drug Ingaron belongs to the group of immunostimulants. The main substance, interferon gamma, is a compound of 144 amino acid residues. It is obtained by microbiological synthesis in a recombinant strain of Escherichia coli and purification using column chromatography.
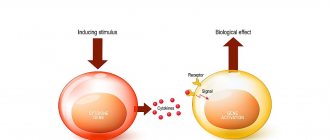
Interferon gamma is a cytokine that has a pronounced anti-inflammatory effect. It is produced naturally in the body by killer cells, T-helper cells, CD4 and CD8 lymphocytes.
Due to the presence of specific receptors on the membranes, cytotoxic T-lymphocytes, neutrophils, macrophages and killer cells are sensitive to this component of Ingaron. Interferon gamma activates their effector functions, including increasing microbicidal activity, stimulating the production of superoxide radicals and cytokines. these substances lead to the death of viral particles. In addition, Ingaron blocks the synthesis of proteins, DNA and RNA of microorganisms.
Interferon gamma inhibits the response of B lymphocytes to interleukin-4, inhibits the production of immunoglobulin E and the expression of the CD23 antigen. It also induces apoptosis of differentiated B cells, from which autoreactive clones can be formed that damage the body's own cells.
Interferon gamma neutralizes the suppressive effect of IL-4 on IL-2-dependent generation and proliferation of killer cells activated by lymphokines. Stimulates the synthesis of acute-phase proteins formed in response to the development of the inflammatory process in the human body. The drug is also able to increase the efficiency of antigen presentation and the ability of T lymphocytes to recognize pathogenic agents.
Ingaron lyophilisate for the preparation of solution for intranases injected 100 thousand IU + water for intranases 5 ml
Release form
Lyophilisate for preparing a solution for intranasal administration.
pharmachologic effect
Pharmacotherapeutic group
Immunomodulatory agent.
ATX code [LO3AB03]
Pharmacological properties
INGARON® is a recombinant human interferon gamma, consisting of 144 amino acid residues (a.a.), lacking the first three a. O. Cys-Tyr-Cys replaced by Met. Molecular weight 16.9 kDa. Obtained by microbiological synthesis in a recombinant strain of Escherichia coli and purified by column chromatography. The specific antiviral activity on cells (human fibroblasts) infected with the vesicular stomatitis virus is 2·107 IU per mg of protein. Interferon gamma (immune interferon) is the most important anti-inflammatory cytokine, the producers of which in the human body are natural killer cells, CD4Th1 cells and CD8 cytotoxic suppressor cells.
Receptors for interferon gamma are found in macrophages, neutrophils, natural killer cells, and cytotoxic T lymphocytes. Activates the effector functions of these cells, in particular their microbicidality, cytotoxicity, their production of cytokines, superoxide and nitrooxide radicals (thereby causing the death of intracellular parasites). Interferon gamma blocks the replication of viral DNA and RNA, the synthesis of viral proteins and the assembly of mature viral particles. At the same time, it causes a cytotoxic effect on virus-infected cells.
Inhibits the B-cell response to interleukin-4, suppresses the production of IgE and the expression of the CD23 antigen. It is an inducer of apoptosis of differentiated B cells, giving rise to autoreactive clones. It reverses the suppressive effect of interleukin-4 on interleukin-2-dependent proliferation and generation of lymphokine-activated killer cells. Activates the production of proteins of the acute phase of inflammation, enhances the expression of genes C2 and C4 components of the complement system.
Unlike other interferons, it increases the expression of MHC antigens of both classes I and II on different cells, and induces the expression of these molecules even on those cells that do not constitutively express them. This increases the efficiency of antigen presentation and the ability of T lymphocytes to recognize them.
Interferon gamma blocks the synthesis of β-TGF, which is responsible for the development of fibrosis of the lungs and liver.
Indications
Prevention and treatment (as part of complex therapy) of influenza. Prevention and treatment (as part of complex therapy) of H5N1 and H1N1 influenza.
Contraindications
Individual intolerance to interferon gamma or any other component of the drug. Pregnancy. Children's age (under 7 years).
Use during pregnancy and breastfeeding
Contraindicated during pregnancy.
Compound
Active substances:
Interferon gamma –100,000 IU (5.5±0.5).
Excipients: mannitol
Description
The drug is a loose or porous mass of white color, hygroscopic.
Directions for use and doses
Intranasally. The contents of the bottle are dissolved in 5 ml of water for injection.
At the first signs of influenza, ARVI, 2 drops in each nasal passage after toileting the nasal passages 5 times a day for 5 - 7 days.
For the prevention of acute respiratory viral infections and influenza in case of contact with a patient and/or hypothermia
2 – 3 drops in each nasal passage every other day 30 minutes before breakfast for 10 days.
If necessary, preventive courses are repeated. For single contact, one instillation is sufficient.
After instillation, it is recommended to massage the wings of the nose with your fingers for several minutes to evenly distribute the drug in the nasal cavity.
Side effects
Not noted.
Storage conditions
In a dry place, protected from light and out of reach of children, at a temperature not exceeding +25o C.
Store the drug solution for no more than 10 days in the refrigerator (do not freeze).
Best before date
2 years.
Conditions for dispensing from pharmacies
Dispensed with a doctor's prescription
Indications and contraindications for use
Ingaron in the form of intranasal drops is indicated for use in the following cases:
- Treatment and prevention of acute respiratory viral infections.
- Treatment and prevention of influenza strains H5N1 and H1N1.
It should be noted that the drug should be used as part of complex therapy for the disease.
Contraindications to the use of Ingaron:
- The presence of increased hypersensitivity to interferon gamma or to auxiliary components (mannitol) of the drug.
- The period of pregnancy and breastfeeding.
- Children's age up to 7 years.
INGARON (intranasal form)
INGARON® (interferon gamma, 100,000 IU)
human recombinant interferon gamma (immune interferon) has an immunostimulating and antiviral effect: it contributes to the formation of cell resistance to the effects of viruses.
Ingaron - effectively stimulates the immune system and provides reliable protection against influenza, including H5N1 and H1N1 influenza
.
The drug is administered intranasally (nasal drops)
- The only interferon gamma drug in the Russian Federation
- Included in the list of vital and essential drugs
- Included in the standards of specialized medical care for influenza of moderate and severe severity, severe acute respiratory viral infections, clinical guidelines for influenza and acute respiratory viral infections
- Active against a wide range of viral infections
- Potentiates the activity of many antiviral drugs used in the complex therapy of ARVI and influenza
- Drug resistance to INGARON® does not develop
- The only interferon gamma drug in the Russian Federation1
- Included in the list of vital and essential drugs 2
- Included in the standards of specialized medical care for influenza of moderate3 and severe4 severity, ARVI of severe severity5, clinical guidelines for influenza and ARVI
- Active against a wide range of viral infections 6
- Potentiates the activity of many antiviral drugs used in the complex therapy of ARVI and influenza 7
- Drug resistance to INGARON® does not develop8
1 According to https://grls.rosminzdrav.ru as of July 20, 2015 2 Appendix No. 1 to the order of the Government of the Russian Federation dated December 30, 2014 No. 2782-r 3 Appendix to the order of the Ministry of Health of the Russian Federation dated November 9, 2012 No. 724 4 Appendix to the order of the Ministry of Health of the Russian Federation dated November 9, 2012 No. 842n 5 Appendix to the order of the Ministry of Health of the Russian Federation dated November 7, 2012 No. 657n 6 Kisilev O.I., Ershov F.I., Deeva E.G. . Interferon-gamma: a new cytokine in clinical practice. Ingaron®. Moscow – St. Petersburg, 2007, p. 51-52. 7 In vitro study of the virus-inhibitory activity of antiviral drugs against influenza virus A/California/07/09 (H1N1), Influenza Research Institute of the Northwestern Branch of the Russian Academy of Medical Sciences, St. Petersburg, 2009. 8 Report on the results of a randomized, placebo-controlled study assessing the effectiveness and tolerability of the drug INGARON® (interferon gamma human recombinant)
Ingaron: instructions for use
The drug is intended for intranasal use.
To prepare the medicinal solution, the lyophilisate is dissolved in 5 milliliters of the solvent contained in the kit (water for injection).
At the first manifestations of the development of influenza or other acute respiratory viral infection, it is recommended to instill 2 drops 4 to 6 times a day into each nasal opening. The course of treatment is from 5 to 7 days.
To prevent infectious viral diseases, the development of which is possible due to general hypothermia or after contact with a sick person, you should use 2 drops of the drug every other day. Preventive course – 10 days. If necessary, the specialist may prescribe reuse of the drug.
To ensure equal distribution of the drug in the nasal cavity, improve its absorption and increase efficiency, it is recommended to massage the side walls and wings of the nose with light movements immediately after instillation.
Ingaron bottle for intranasal administration 100000 IU N1
A country
Russia
The country of production may vary depending on the batch of goods. Please check with the operator for detailed information when confirming your order.
Active substance
Interferon gamma
Compound
The active substance is interferon gamma.
pharmachologic effect
Ingaron is a recombinant human interferon gamma. It is the most important anti-inflammatory cytokine, the producers of which in the human body are natural killer cells, CD4 Th1 cells and CD8 cytotoxic suppressor cells. Receptors for interferon gamma are found in macrophages, neutrophils, natural killer cells, and cytotoxic T lymphocytes. Activates the effector functions of these cells, in particular their microbicidality, cytotoxicity, their production of cytokines, superoxide and nitrooxide radicals (thereby causing the death of intracellular parasites). Interferon gamma blocks the replication of viral DNA and RNA, the synthesis of viral proteins and the assembly of mature viral particles. At the same time, it causes a cytotoxic effect on virus-infected cells. Inhibits the B-cell response to interleukin-4, suppresses the production of IgE and the expression of the CD23 antigen. It is an inducer of apoptosis of differentiated B cells, giving rise to autoreactive clones. It reverses the suppressive effect of interleukin-4 on interleukin-2-dependent proliferation and generation of lymphokine-activated killer cells. Activates the production of proteins of the acute phase of inflammation, enhances the expression of genes C2 and C4 components of the complement system. Unlike other interferons, it increases the expression of MHC antigens of both the first and second classes on different cells, and induces the expression of these molecules even on those cells that do not express them constitutively. This increases the efficiency of antigen presentation and the ability to recognize them by T lymphocytes. Interferon gamma blocks the synthesis of β-TGF, which is responsible for the development of fibrosis of the lungs and liver.
Indications for use
• Prevention and treatment (as part of complex therapy) of influenza. • Prevention and treatment (as part of complex therapy) of avian influenza.
Mode of application
Intranasally. The contents of the bottle are dissolved in 5 ml of water for injection. At the first signs of ARVI, influenza: 2 drops in each nasal passage after toileting the nasal passages 5 times a day for 5-7 days. For the prevention of ARVI and influenza: upon contact with a patient and/or for hypothermia, 2-3 drops in each nasal passage every other day, 30 minutes before breakfast for 10 days. If necessary, preventive courses are repeated. For single contact, one instillation is sufficient. After instillation, it is recommended to massage the wings of the nose with your fingers for several minutes to distribute the drug evenly in the nasal cavity.
Interaction
No data.
Side effect
No data.
Contraindications
Individual intolerance to interferon gamma or any other component of the drug, pregnancy, children (under 7 years).
Overdose
No data.
special instructions
No data.
Additional Information
The shelf life of the drug is 24 months from the date of manufacture and subject to the manufacturer’s recommendations. The medicine should be stored in a dry place, out of reach of direct sunlight. Temperature – no more than 25 degrees. Children's access to the drug should also be limited.
After preparing the intranasal solution, it can be stored at a temperature of 0 to 10 degrees (in the refrigerator, without freezing) for no more than 10 days. This is explained by the fact that the drops contain no preservatives.
You can purchase the medication without a doctor's prescription, but before starting treatment you should consult a specialist.