Reaferon-ES-Lipint® Lyophilisate, bottle, 6 pcs., 500,000 IU, for suspension preparation
Directions for use and doses
It is administered orally. Immediately before use, add 1-2 ml of distilled or cooled boiled water to the contents of the bottle. Shaking for 1-5 minutes should form a homogeneous suspension. For acute hepatitis B, the drug is taken 30 minutes before meals according to the following scheme:
- adults and school-age children - but 1 million IU 2 times a day for 10 days;
- children of preschool age (from 3 to 7 years) - 500 thousand IU 1 time / day for 10 days or. after control biochemical blood tests, for a longer period until complete clinical recovery.
For chronic hepatitis B in active and inactive replicative forms, as well as for chronic hepatitis B associated with glomerulonephritis, the drug is taken 30 minutes before meals according to the following scheme:
- adults and school-age children - 1 million IU 2 times/day for 10 days and then for 1 month - every other day, 1 time/day (at night);
- children of preschool age (from 3 to 7 years) - but 500 thousand IU 2 times / day for 10 days and then - 500 thousand IU for 1 month every other day, 1 time / day (at night).
When carrying out specific immunotherapy, the drug is taken in the morning, 30 minutes after meals. according to the following scheme:
- for allergic rhinoconctivitis in adults - 500 thousand IU daily for 10 days (course dose 5 million IU);
- for atonic bronchial asthma in adults - but 500 thousand IU 1 time / day for 10 days, and then 500 thousand IU every other day for 20 days. The total duration of treatment is 30 days.
For the prevention and treatment of influenza and ARVI, the drug is taken 30 minutes before meals:
- for prevention: adults and children over 15 years old - 500 thousand IU 1 time / day 2 times a week for 1 month during an increase in incidence; children from 3 to 15 years old, 250 thousand IU 1 time / day, 2 times a week for 1 month during an increase in incidence;
- for the treatment of influenza and ARVI: adults and children over 15 years old - 500 thousand IU daily 2 times a day for 3 days: children from 3 to 15 years old - 250 thousand IU daily 2 times a day for 3 days.
For complex therapy of urogenital infections in adults, the drug is taken 30 minutes before meals, 500 thousand IU daily, 2 times a day for 10 days. For complex therapy of tick-borne encephalitis, the drug is taken 30 minutes before meals:
- for febrile form: 500 thousand IU 2 times a day (morning and evening) for 7 days;
- for the meningeal form: 500 thousand IU 2 times a day (morning and evening) for 10 days.
For emergency prevention of tick-borne encephalitis, the drug is taken 30 minutes before meals, 500 thousand IU 2 times a day (morning and evening) for 5 days. Anti-tick immunoglobulin is administered intramuscularly once no later than the 4th day after the tick bite at a dose of 0.1 ml/kg.
Instructions for use of the drug Reaferon-LIPINT®
Active substance: liposomal form of interferon alpha-2b
Dosage form: capsules
Composition: one capsule contains: active substance – human recombinant interferon alpha-2b – 500,000 IU; excipients: sodium chloride – 8.01 mg, sodium phosphate disubstituted 12-water (sodium hydrogen phosphate dodecahydrate) – 4.52 mg, sodium phosphate disubstituted 2-water (sodium dihydrogen phosphate dihydrate) – 0.56 mg, Lipoid C 100 (phosphatidylcholine ) – 41.18 mg, cholesterol – 4.53 mg, alpha-tocopherol acetate (vitamin E) – 0.56 mg, lactose monohydrate – 91.34 mg, colloidal anhydrous silicon dioxide – 7.54 mg (not more than 5% ); capsule composition (body and lid): titanium dioxide (E 171) – 2%, gelatin – up to 100%.
Description: white hard gelatin capsules. The contents of the capsules are fine-crystalline powder, white or white with a yellowish tint. Hygroscopic.
Pharmacotherapeutic group: MIBP - cytokine.
ATX code : L03AB05.
Pharmacological and immunobiological properties
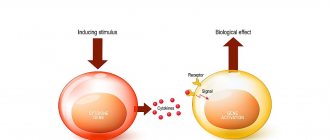
The drug has an immunomodulatory and antiviral effect.
Human recombinant interferon alpha-2b, which is the active ingredient in the drug, is synthesized by bacterial cells of the Escherichia coli strain SG-20050/pIF16, into the genetic apparatus of which the human interferon alpha-2b gene is integrated. It is a protein containing 165 amino acids and is identical in characteristics and properties to human leukocyte interferon alpha-2b.
The antiviral effect of interferon alpha-2b manifests itself during the period of virus reproduction through active inclusion in the metabolic processes of cells. Interferon, interacting with specific receptors on the surface of cells, initiates a number of intracellular changes, including the synthesis of specific cytokines and enzymes (2-5-adenylate synthetases and protein kinases), the action of which inhibits the formation of viral protein and viral ribonucleic acid in the cell.
The immunomodulatory effect of interferon alpha-2b is manifested in an increase in the phagocytic activity of macrophages, an increase in the specific cytotoxic effect of lymphocytes on target cells, a change in the quantitative and qualitative composition of secreted cytokines; changes in the functional activity of immunocompetent cells; changes in the production and secretion of intracellular proteins.
Indications for use
Treatment of influenza and other acute respiratory viral diseases in adults as part of complex therapy.
Prevention of influenza and other acute respiratory viral diseases in adults during epidemics and seasonal increases in incidence.
Contraindications
- children under 18 years of age;
- pregnancy; - lactation period;
- lactase deficiency, lactose intolerance, glucose-galactose malabsorption syndrome;
- hypersensitivity to interferon or any other components of the drug.
Carefully
Renal and/or liver failure, severe myelosuppression.
Use during pregnancy and breastfeeding
The drug is contraindicated for use during pregnancy and lactation.
Directions for use and doses
The drug is taken orally, 30 minutes before meals.
When treating influenza and ARVI - 500,000 IU (1 capsule) daily, 2 times a day for 5 days.
For the prevention of influenza and ARVI - 500,000 IU (1 capsule) per day, 2 times a week for a month.
If swallowing is difficult, carefully open the capsules and take the contents with a small amount of water.
Side effect
Possible allergic reactions, flu-like syndrome (chills, fever, fatigue, lethargy, headache, myalgia, arthralgia, loss of appetite).
Overdose
There were no cases of drug overdose. Increased dose-dependent side effects are possible. Treatment is symptomatic.
Interaction with other drugs
Interferon alpha is capable of reducing the activity of P-450 cytochromes and, therefore, influencing the metabolism of cimetidine, phenytoin, chimes, theophylline, diazepam, propranolol, warfarin, and some cytostatics. May enhance the neurotoxic, myelotoxic or cardiotoxic effects of drugs prescribed previously or simultaneously with it. Co-administration with drugs that depress the central nervous system and immunosuppressive drugs (including oral and parenteral forms of corticosteroids) should be avoided.
Drinking alcohol during treatment is not recommended.
special instructions
If allergic reactions occur, you should consult a doctor.
Impact on the ability to drive vehicles and machinery
The use of the drug does not affect the ability to drive a vehicle or operate potentially dangerous machinery.
Release form
Capsules, 500,000 IU.
10 or 20 capsules in bottles made of high-density polyethylene (HDPE), sealed with a LDPE cap.
1 bottle along with instructions for use in a cardboard box.
Storage conditions
Store in a place protected from light, at a temperature not exceeding 8 °C.
Keep out of the reach of children.
Best before date
2 years.
Cannot be used after the expiration date indicated on the packaging.
Vacation conditions
Available without a prescription.
Manufacturer
JSC "Vector-Medica" Legal address: 630099, Russia, Novosibirsk, st. M. Gorkogo, 17a, tel./fax: (383) 363-32-96; production address: 630559, Novosibirsk region, r.p. Koltsovo, bldg. 13, 15.
Reaferon-LIPINT capsules 500 thousand IU No. 10
A country
Russia
The country of production may vary depending on the batch of goods. Please check with the operator for detailed information when confirming your order.
Compound
1 capsule contains: human recombinant interferon alpha-2b 500,000 IU.
Excipients: sodium chloride - 8.01 mg, sodium phosphate, disubstituted, 12-hydrate (sodium hydrogen phosphate dodecahydrate) - 4.52 mg, sodium dihydrogen phosphate dihydrate, sodium phosphate dihydrate, monosubstituted 2-water - 0.56 mg, lactose monohydrate - 91.34 mg, colloidal silicon dioxide anhydrous - 7.54 mg (no more than 5%). Composition of the capsule shell (body and lid): titanium dioxide (E171) - 2%, gelatin - up to 100%. Hard gelatin capsules, white. The contents of the capsules are fine-crystalline hygroscopic powder, white or white with a yellowish tint.
pharmachologic effect
The drug has an immunomodulatory and antiviral effect. Human recombinant interferon alpha-2b, which is the active ingredient in the drug, is synthesized by bacterial cells of the Escherichia coli strain SG-20050/plF16, in the genetic apparatus of which the human interferon alpha-2b gene is integrated. It is a protein containing 165 amino acids and is identical in characteristics and properties to human leukocyte interferon alpha-2b. The antiviral effect of interferon alpha-2b manifests itself during the period of virus reproduction through active inclusion in the metabolic processes of cells. Interferon, interacting with specific receptors on the surface of cells, initiates a number of intracellular changes, including the synthesis of specific cytokines and enzymes (2-5-adenylate synthetase and protein kinase), the action of which inhibits the formation of viral protein and viral ribonucleic acid in the cell. The immunomodulatory effect of interferon alpha-2b is manifested in an increase in the phagocytic activity of macrophages, an increase in the specific cytotoxic effect of lymphocytes on target cells, a change in the quantitative and qualitative composition of secreted cytokines; changes in the functional activity of immunocompetent cells; changes in the production and secretion of intracellular proteins.
Indications for use
— treatment of influenza and other acute respiratory viral diseases in adults as part of complex therapy; - prevention of influenza and other acute respiratory viral diseases in adults during epidemics and seasonal increases in incidence.
Side effects
Possible allergic reactions, flu-like syndrome (chills, fever, fatigue, lethargy, headache, myalgia, arthralgia, loss of appetite).
Contraindications
- renal and/or liver failure;
- severe myelosuppression. Use during pregnancy and lactation The drug is contraindicated for use during pregnancy and lactation.
Mode of application
The drug is taken orally, 30 minutes before meals. When treating influenza and ARVI - 500,000 IU (1 capsule) daily 2 times a day for 5 days. For the prevention of influenza and ARVI - 500,000 IU (1 capsule) per day, 2 times a week for a month. If swallowing is difficult, carefully open the capsules and take the contents with a small amount of water.
special instructions
If allergic reactions occur, you should consult a doctor. Effect on the ability to drive vehicles and machinery The use of the drug does not affect the ability to drive a vehicle or potentially dangerous machinery.
Overdose
There were no cases of drug overdose. Increased dose-dependent side effects are possible. Treatment is symptomatic.
Interaction with other drugs
Interferon alpha is able to reduce the activity of P450 cytochromes and, therefore, affect the metabolism of cimetidine, fepitoip, chimes, theophylline, diazepam, propranolol, warfarin. some cytostatics. May enhance neurotoxicity. myelotoxic or cardiotoxic effect of drugs prescribed previously or simultaneously with it. Co-administration with drugs that depress the central nervous system and immunosuppressive drugs (including oral and parenteral forms of corticosteroids) should be avoided. Drinking alcohol during treatment is not recommended.