Compound
- 1 ml of Nazivin Sensitive in the form of drops for children contains 100 mcg of oxymetazoline hydrochloride . Additional substances: citric acid monohydrate, glycerol 85%, water, sodium citrate dihydrate.
- 1 ml of Nazivin Sensitive in the form of a spray for children contains 250 mcg of oxymetazoline hydrochloride. One dose contains 11.25 mcg of oxymetazoline hydrochloride . Additional substances: citric acid monohydrate, glycerol 85%, water, sodium citrate dihydrate.
Release form
- Nasal drops 0.01% is a transparent solution of pale yellow color, 5 ml of solution in a polyethylene bottle with a dispenser, one bottle in a paper box.
- Nasal spray is a transparent solution of pale yellow color, 10 ml of solution (includes 220 doses) in a polyethylene bottle with a dispenser, one bottle in a paper box.
Note: in numerous descriptions and references to these medicines on the Internet you can find such names as “Nazivin for children”, “Nazivin for baby”, “Nazivin for babies”, “Nazivin for children under one year old”. Officially, there are no drugs with these names. The first name includes both described forms of the drug: spray used in children 1-6 years old and drops used in children under one year of age. And the rest of the listed names, including “Nazivin for babies”, “Nazivin for children up to one year old”, correspond only to the drug in the form of drops.
When is Nazivin shown?
It is necessary to use the drug to eliminate the symptoms of nasal breathing disorders:
- with a runny nose caused by an allergic reaction, infectious inflammatory processes during ARVI;
- with complications of otitis media;
- for sinusitis and inflammation of the Eustachian tubes.
Indications for the use of the drug include preparation for diagnostic and therapeutic ENT procedures to eliminate edema.
Pharmacodynamics and pharmacokinetics
Pharmacodynamics
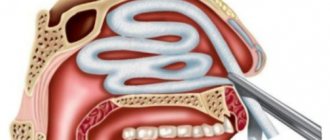
Local vasoconstrictor drug. When applied to the inflamed mucous membranes of the nasal cavity, it significantly reduces its swelling and the volume of nasal discharge. Normalizes nasal breathing. Reducing swelling of the nasal mucosa helps restore ventilation of the paranasal sinuses and middle ear spaces, which reduces the likelihood of infectious complications ( sinusitis, sinusitis, otitis media ). When applied locally intranasally in therapeutic concentrations, it does not cause hyperemia or irritation of the mucous membranes.
When used intranasally, topical oxymetazoline has no systemic effect.
Pharmacokinetics
The active substance begins to act in 1-2 minutes. The duration of action of the drug reaches 12 hours. The half-life of oxymetazoline when applied topically in the nasal cavity is 34-36 hours. About 2.1% of the dose is evacuated in the urine, 1.1% in feces.
Mechanism of action
Oxymetazoline in the medication affects the capillary network of the nasal mucosa, causing the walls of blood vessels to narrow. As a result, microcirculation improves, swelling is eliminated, the accumulation of exudate is reduced, and the risk of numerous complications of rhinitis in children, including acute otitis and sinusitis, is reduced.
The product begins to act after 1–2 minutes of contact with the nasal sinuses: normal breathing is restored and the outflow of mucous secretions improves. Oxymetazoline penetrates into the bloodstream in small quantities, but its tiny concentration does not allow the development of any systemic reactions and does not cause harm to the body. The effect of one dose of Nazivin lasts up to 10–12 hours. The components of the drug are excreted in the urine and intestinal contents within 1–1.5 days. They do not accumulate in body tissues. Proper use of the product does not lead to irritation of the mucous membrane or increased symptoms.
Nazivin does not affect the general course of the disease and its causes. It is used only to eliminate the main sign of pathology and restore breathing.
Contraindications
- angle-closure glaucoma;
- hypersensitivity to the components of the drug;
- atrophic rhinitis;
- age less than 1 year (for all forms of the drug except Nazivin Sensitive in the form of drops);
- age less than 6 years (for the drug in the form of a spray with a dosage of 500 mcg/ml).
It is necessary to prescribe the drug with caution in case of increased intraocular pressure, arterial hypertension , heart failure or chronic renal failure, angina pectoris, pheochromocytoma , severe atherosclerosis , arrhythmia , prostatic hyperplasia with signs of urinary retention, diabetes mellitus , hyperthyroidism , as well as in persons taking MAO inhibitors over the past 2 weeks (and another 2 weeks after stopping their use), Bromocriptine , tricyclic antidepressants .
Contraindications and side effects
A nasal vasoconstrictor should not be used to treat children suffering from angle-closure glaucoma and other severe eye lesions. Nazivin is also prohibited in the case of atrophic rhinitis, when individual intolerance to its components occurs. In addition, the product in the form of a spray should not be used in pediatric patients under 1 year of age.
Nazivin requires careful use in case of increased intraocular, intracranial and blood pressure, diabetes mellitus, thyroid disorders, heart failure, inflammation and decreased renal function, and delayed diuresis.
When using the medicine, negative reactions of the body are possible:
- sneezing;
- increased nasal discharge;
- lacrimation;
- dry mucous membranes.
In most cases, the drug does not require discontinuation, since undesirable symptoms disappear on their own within one day or some time after using Nazivit.
With prolonged or improper use of the drug, increased nasal congestion and the formation of drug dependence are likely. Occasionally, Nazivin causes heart rhythm disturbances, increased sweating, anxiety, restlessness and sleep disturbances. During the treatment period, children may have a decreased appetite and become more capricious.
Side effects
- Local reactions: sneezing, burning of the nasal mucous membranes, blurred vision in case of accidental contact with the eyes, dry nasal mucous membranes, a feeling of stuffiness ( reactive hyperemia ) after the effect of using the drug disappears. Long-term continuous use of vasoconstrictors can lead to atrophy of the mucous membranes of the nasal cavity, tachyphylaxis , and repeated swelling of the mucous membranes of the nasal cavity ( rhinitis of drug etiology).
- Reactions from the circulatory system: increased blood pressure, tachycardia , palpitations.
- Reactions from nervous activity: dizziness, sleep disturbance, headache, anxiety, insomnia , irritability.
- Other reactions: exanthema , nausea.
Nazivin, nasal drops 0.01% 5 ml
Manufacturer
Merck KGaA, Russia
Compound
1 ml of the drug contains: Active substance: Nazivin 0.01% - Oxymetazoline hydrochloride 0.1 mg Nazivin 0.025% - Oxymetazoline hydrochloride 0.25 mg Nazivin 0.05% - Oxymetazoline hydrochloride 0.5 mg Excipients: benzalkonium chloride 50% solution , disodium edetate dihydrate, sodium dihydrogen phosphate dihydrate, sodium hydrogen phosphate dihydrate, sodium hydroxide 1 M solution, purified water.
pharmachologic effect
Nazivin® (oxymetazoline) has a vasoconstrictor effect. When applied topically to the inflamed nasal mucosa, it reduces swelling and nasal discharge. Restores nasal breathing. Elimination of mucosal edema helps restore aeration of the paranasal sinuses and middle ear cavity, which prevents the development of bacterial complications (sinusitis, sinusitis, otitis media). When used locally intranasally in therapeutic concentrations, it does not irritate the mucous membrane and does not cause hyperemia. When administered locally intranasally, the drug does not have a systemic effect. The drug begins to act quickly (within a few minutes). The duration of action of Nazivin is up to 12 hours.
Indications
- treatment of acute respiratory diseases accompanied by a runny nose;
- allergic rhinitis;
- vasomotor rhinitis;
- to restore drainage in case of inflammation of the paranasal sinuses, eustachitis, otitis media;
- to eliminate swelling before diagnostic manipulations in the nasal passages.
Use during pregnancy and breastfeeding
When used during pregnancy or breastfeeding, do not exceed the recommended dosage. The drug should be used only after a careful assessment of the risk-benefit ratio for the mother and fetus.
Contraindications
Atrophic rhinitis; angle-closure glaucoma; hypersensitivity to the components of the drug. You should adhere to the recommended concentrations of the drug intended for different age categories (see methods of use). With caution In patients taking monoamine oxidase inhibitors and other drugs that increase blood pressure for up to 10 days after their use; with increased intraocular pressure, during pregnancy and lactation, with severe forms of cardiovascular diseases (hypertension, angina); with thyrotoxicosis and diabetes mellitus.
Side effects
Sometimes: burning or dryness of the nasal membranes, sneezing. In rare cases: after the effect of using Nazivin® wears off, a strong feeling of “stuffiness” in the nose (reactive hyperemia). Repeated overdose with local nasal use sometimes leads to systemic sympathomimetic effects such as increased heart rate (tachycardia) and increased blood pressure. In very rare cases, anxiety, insomnia, fatigue, headaches and nausea have been observed. Long-term continuous use of vasoconstrictor drugs can lead to tachyphylaxis, atrophy of the nasal mucosa and recurrent swelling of the nasal mucosa (rhinitis medicamentosa).
Interaction
When MAO blockers and tricyclic antidepressants are prescribed simultaneously, blood pressure increases. Co-administration of other vasoconstrictor drugs increases the risk of side effects.
How to take, course of administration and dosage
Nazivin® 0.01%, 0.025% and 0.05% drops are intended for use in the nose. Adults and children over 6 years of age: use Nazivin® 0.05% drops, 1 - 2 drops in each nasal passage 2 - 3 times a day. Children from 1 year to 6 years: use Nazivin® 0.025% drops, 1 - 2 drops in each nasal passage 2 - 3 times a day. Children under 1 year: children under 4 weeks of age are prescribed 1 drop of Nazivin® 0.01% in each nasal passage 2 - 3 times a day. From the 5th week of life to 1 year - 1 - 2 drops in each nasal passage 2 - 3 times a day. To ensure dosage accuracy, the bottle of Nazivin® 0.01% drops has a graduated pipette with marks on the number of drops. For example, if 1 drop is prescribed, then the pipette should be filled with the solution to the 1 mark. The following procedure has also been proven effective: depending on the age, 1 - 2 drops of a 0.01% solution are applied to cotton wool and wiped the nasal passages. Nazivin® 0.01%, 0.025% and 0.05% drops should be used for 3 to 5 days. Doses higher than recommended can only be used under medical supervision.
Overdose
Symptoms (after a significant overdose or accidental ingestion): constriction of the pupils, nausea, vomiting, cyanosis, increased body temperature, tachycardia, arrhythmia, vascular insufficiency, arterial hypertension, respiratory disorders, pulmonary edema, cardiac arrest;
in addition, mental disorders may appear, as well as depression of central nervous system functions, accompanied by drowsiness, decreased body temperature, bradycardia, arterial hypotension, respiratory arrest and possible development of coma. Treatment: gastric lavage, taking activated carbon and urgently consulting a doctor.
Special instructions
Long-term use and overdose of the drug should be avoided, especially in children. After long-term use or administration of cold remedies containing oxymetazoline in dosages exceeding the recommended ones, a general effect on the cardiovascular system and central nervous system cannot be excluded. In these cases, the ability to operate a vehicle or equipment may be impaired.
Release form
Nasal drops
Storage conditions
In a place protected from light, at a temperature not exceeding 25 °C.
Best before date
3 years
Active substance
Oxymetazoline
Dosage form
nasal drops
Purpose
For children from birth
Indications
Sinusitis, Flu, Colds, Runny nose, Otitis media, Hay fever, Allergic rhinitis
Barcode and weight
Barcode: 4054839084331, 4054839472589, 4022536645612 Weight: 0.033 kg
Instructions for children's Nazivin Sensitive (Method and dosage)
All described forms of release of the drug are used intranasally.
Instructions for drops for children (Nazivin baby)
The age for using the drug in the form of Nazivin drops for children is up to one year.
Children under 4 weeks of age are recommended to take 1 drop of the product in each nostril up to three times a day. Children from the fifth week of life to 12 months of age are prescribed to instill 1-2 drops of the medicine into each nostril up to three times a day.
1 drop of the drug in this release form includes 2.8 mcg of oxymetazoline hydrochloride . Before use, the bottle must be turned over and the product should be instilled, tilting the sick child's head back.
The following application has also been proven effective: apply 1-2 drops of Nazivin Sensitive to cotton wool (depending on the patient’s age) and wipe each nasal passage from the inside.
At the recommended dose, the medicine can be used for up to a week without a doctor’s prescription. If the symptoms of the disease intensify or there are no signs of improvement within 3 days, you should consult a doctor. In doses exceeding those recommended, the drug is allowed to be used only under the supervision of the attending physician.
Instructions for use of baby spray
One 45 µl spray contains 11.25 µg of oxymetazoline hydrochloride .
A spray with a dosage of 11.25 mcg/dose is used in children 1-6 years old: spray 1 dose of the product in each nasal passage up to three times a day. The drug is used for 5-7 days. The drug can be re-prescribed only after a few days.
Precautionary measures
Long-term use and overdose of the drug should be avoided, especially in children.
Impact on the ability to drive vehicles and operate machinery
After long-term use or administration of cold remedies containing oxymetazoline in dosages exceeding the recommended ones, a general effect on the cardiovascular system and central nervous system cannot be excluded. In these cases, the ability to operate a vehicle or equipment may be impaired.
Interaction
The medicine slows down the absorption of local anesthetics and increases their duration of action.
When using Nazivin Sensitive for 2 weeks before using MAO inhibitors and for 2 weeks after completing their use, as well as during therapy with tricyclic antidepressants or other drugs that provoke an increase in blood pressure , a noticeable increase in blood pressure is observed.
Simultaneous administration of other vasoconstrictors increases the risk of adverse reactions.
special instructions
Avoid contact of this product with eyes.
It is recommended to use the drug individually to avoid the spread of a viral or bacterial infection.
After prolonged use of drugs for the common cold, including oxymetazoline in large doses, the appearance of effects on cardiovascular and nervous activity cannot be ruled out. In these cases, patients should be careful when driving.
Overdose
After a significant overdose or accidental ingestion, the following symptoms may appear: constriction of the pupils, nausea, vomiting, cyanosis, fever, tachycardia, arrhythmia, collapse, cardiac depression, arterial hypertension, pulmonary edema, respiratory disorders. In addition, mental disorders may appear, as well as depression of the functions of the central nervous system, accompanied by drowsiness, decreased body temperature, bradycardia, arterial hypotension, respiratory arrest and possible development of coma.
In case of overdose associated with oral administration of the drug, gastric lavage and activated charcoal are prescribed.
Analogs
Level 4 ATX code matches:
Xymelin Eco
Xymelin
Nazivin
Galazolin
Lazorin
Otrivin
Naphthyzin
Sanorin
Knoxprey
For the nose
Lazolvan Rino
Afrin
Rhinorus
Eucazoline Aqua
Rinazolin
Grippostad Reno
Farmazolin
Xylometazoline
Nazol Advance
Nazol Baby
Vicks Active Sinex, Afrin Extra, Nazol Advance, 4-Way, Nazol Baby, Afrin, Nazol Kids, Nazospray, Nesopin, Oxymetazoline, Noxprey, Nazol, Fazin, Sanorinchik, Fervex.