Iron is responsible for many processes, among the main ones are the delivery of oxygen to organs, regulation of respiration, and metabolism. All the most important things about the mineral are in this article.
The material was commented on and checked by Natalya Polenova, family doctor, cardiologist and nutritionist at GMS Clinic
The body does not produce iron on its own; a person receives 1-2 mg of this element with food. Each of us loses approximately the same amount through dead skin and intestinal cells.
Why is there a shortage?
The most common cause is blood loss, for example due to injury or bowel disease, or due to excessive menstrual bleeding.
“Iron deficiency underlies more than half of anemia,”
says Ph.D.
cardiologist and nutritionist, GMS Clinic Natalya Polenova. “The main causes of iron deficiency: unbalanced diet, malabsorption, cyclic blood loss in women of reproductive age, donation, pregnancy, lactation, rapid growth during puberty.”
The second reason is poor absorption of iron. This can occur with medications, chronic bowel disease, inflammation, or genetic disorders.
A hematologist at the Federal State Budgetary Institution “National Medical Research Center of Hematology” of the Ministry of Health of Russia clarifies that iron deficiency conditions occur in many diseases with malabsorption, ranging from serious cancer to helminthiasis and the presence of Helicobacter pylori bacteria, which absorb iron.
Excess iron
An overdose of iron in a healthy person is quite difficult to achieve as part of a normal diet. Iron is a rather ungrateful mineral component and is only absorbed to a small extent.
Additionally, many other food ingredients can effectively prevent this. We are talking about black tea, coffee, products containing calcium, phytic acids, fiber or polyphenols, which are almost impossible to get rid of.
This looks a little different with iron supplements. A large dose of the element is concentrated into a small tablet that is easy to swallow. Supplement overdose can result from taking too much or too many different medications, most of which contain iron.
In a healthy person, a situation in which the body cannot cope with excessive iron intake is unlikely, including due to natural protective mechanisms. This is an HFE protein that blocks the absorption of iron in the intestines when levels are high. Specific genes are responsible for its production.
Unfortunately, there are people for whom these mechanisms do not work properly. These people suffer from hemochromatosis.
Hemochromatosis is a genetically determined disease. Serious symptoms occur when there is a significant excess of iron in the tissues and hemochromatosis is detected only after 30-40 years. In women, the first symptoms of the disease and its detection may occur even later.
How to find out if you have anemia
Typically, in the initial stages, iron deficiency states do not appear externally. But minor symptoms may appear, which are usually ignored and lead to further development of the disease.
“With a lack of iron, the formation of the protein that carries oxygen to cells—hemoglobin—is disrupted. When his performance decreases slightly, fatigue increases, shortness of breath, dizziness and fainting occur with minor exertion,” says the hematologist.
You can find out about a lack of iron in the body only through a blood test. It makes sense to take it if all the symptoms appear for no apparent reason.
The list of tests must include a test for hemoglobin level, hematocrit, mean erythrocyte volume (MCV) and mean erythrocyte hemoglobin content (MCH).
Natalya Polenova notes that a single general blood test assessing hemoglobin levels is often not enough, especially for women or people with chronic heart failure.
Only a doctor can, after studying the tests, identify the cause of the deficiency and prescribe treatment.
Manifestations of iron deficiency anemia
Manifestations of anemia consist of 2 main syndromes: sideropenic and general anemic.
Sideropenic syndrome manifests itself:
- Seizures in the corners of the mouth;
- Glossitis - characterized by a feeling of pain, distension in the tongue, redness of its tip, and subsequently atrophy of the papillae;
- Inflammation of the red border of the lips;
- Dystrophic changes in the skin and its appendages (dry and pale skin, premature wrinkles, brittle nails and hair, the appearance of small cracks);
- Difficulty and painful swallowing of dense foods;
- Muscle weakness;
- Perversion of taste (there is a desire to eat inedible objects - chalk, tooth powder, coal, clay, sand, raw dough, minced meat);
- Perversion of the sense of smell (attraction to the smells of gasoline, kerosene, acetone, varnish, paints, dampness);
- Blue sclera syndrome;
- A pronounced predisposition to acute respiratory viral infections and other infectious and inflammatory processes.
General anemic syndrome manifests itself when the level of hemoglobin and red blood cells decreases.
Who should take iron and how?
No one without a doctor's approval. Vitamins and dietary supplements with extra iron can be dangerous. For example, in people with impaired iron metabolism, excess iron in the diet leads to the development of liver cirrhosis, diabetes mellitus and cardiomyopathies.
“Iron overload due to thoughtless use of drugs leads to very dangerous consequences. In practice, several times we encountered patients with iron overload. Their liver is more like a metal basin,” says the hematologist.
If you have iron deficiency anemia, a simple diet or dietary supplements will not be enough. Such serious conditions are treated with iron injections and medications.
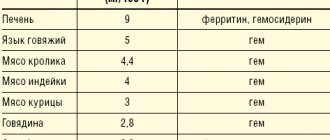
If the doctor sees a slight iron deficiency in the tests, then it is best to regulate it by adding foods containing iron. In food it can be heme - easily digestible and non-heme - difficult to digest. Heme iron is found in meat, poultry, fish and seafood. It is easily digestible and does not depend on other nutritional factors.
“The main sources of dietary iron are red meat and organ meats, especially liver,”
says Natalya Polenova.
— They contain a lot of iron, and it is well absorbed.
Next on the list are fish and seafood, especially sardines, tuna, shrimp, mussels and clams. In addition to the fish itself, quite a lot of iron is contained in its caviar.” Non-heme iron is found mainly in plant foods. But in order for the body to absorb it, it needs the help of organic acids, primarily ascorbic acid.
Polenova clarifies that among plant foods, the most iron is found in beets, asparagus, cauliflower and white cabbage, and spinach. But for successful absorption you will need, for example, citrus juices. When compiling a diet, you need to take into account that 10–15% of iron is absorbed from food.
Its manifestations:
- Pale, slight yellowness of the hands and nasolabial triangle;
- Shortness of breath, increased heart rate, fainting (especially with a rapid transition from a horizontal to a vertical position);
- Palpitations, chest pain;
- Increased fatigue;
- Weakness, dizziness, flashing “spots” before the eyes, drowsiness during the day and insomnia at night, impaired concentration, decreased memory, performance, irritability, tearfulness;
- Loss of appetite, nausea, flatulence;
- Swelling of the face in the morning.
Patients often get used to their condition, regarding it as overwork, stress, etc.
Important to remember
- The causes of iron deficiency are blood loss, malabsorption, unbalanced diet, pregnancy, and helminthiasis.
- To identify a deficiency, you need to take a blood test for hemoglobin level, hematocrit, mean erythrocyte volume (MCV) and mean erythrocyte hemoglobin content (MCH).
- If there is no deficiency, then you should not take iron supplements. It may be dangerous.
- It is best to obtain iron from animal foods, in which it is in heme form.
- If you do not eat animal foods, you will need foods high in vitamin C to absorb plant non-heme iron.
Complications of pregnancy with iron deficiency anemia
Anemia in pregnant women is a pathological background that contributes to the development of a number of complications of pregnancy and childbirth.
- Premature abruption of a normally located placenta, anomalies of labor.
- Increased blood loss during childbirth and bleeding - in 10% of women.
- In 8–12% of cases, the postpartum period is complicated by purulent-septic diseases and uterine subinvolution.
- A lactation disorder was detected, and both quantitative and qualitative changes in breast milk were observed.
- Adverse effects on the condition of the fetus: contributes to the development of fetal growth retardation syndrome, chronic fetal hypoxia.
- A decrease in adaptation of newborns in the early neonatal period is recorded in half of the cases.
References
- Federal clinical guidelines for the diagnosis and treatment of iron deficiency anemia, 2015. - 58 p.
- Lukina, E.A., Smetanina, N.S., Tsvetaeva, N.V. and others. Iron overload: diagnosis and treatment. National clinical guidelines, 2021. - 14 p.
- Rumyantsev, A.G., Maschan, A.A., Chernov, V.M. Federal clinical guidelines for the diagnosis and treatment of iron deficiency anemia, 2015. - 43 p.
- Zaiko, N.N., Byts, Yu.V. Pathological physiology. M.: Medpress, 2008. - 640 p.
- Ganz, T., Nemeth, E. Iron metabolism: interactions with normal and disordered erythropoiesis. Cold Spring Harb Perspect Med., 2012. - Vol. 2(5).
- Chaparro, C., Suchdev, P. Anemia epidemiology, pathophysiology, and etiology in low- and middle-income countries. Ann NY Acad Sci., 2021. - Vol. 1450(1). — P. 15-31.
Functions of iron in the body
Iron is the main component of hemoglobin
Main functions:
- Transport and storage of oxygen. The composition of red blood cells, whose task is to transport gases, includes hemoglobin. This protein contains four iron atoms that bind oxygen. As a result, oxygen is delivered to all organs and tissues and carbon dioxide is transported to the lungs.
- Iron is necessary for the functioning of the thyroid gland, which is responsible for metabolic processes.
- This trace element is associated with the transport of oxygen to cytochromes, which are energy protein molecules.
- Participation in the synthesis of hormones.
- Accelerates the growth processes of the body, nerve fibers, and ensures normal brain function.
- Iron is necessary for proteins and enzymes that are responsible for hematopoiesis, metabolism, cholesterol metabolism and much more.
- Affects the immune system in a certain way.
- Neutralizes toxins.
- Prevention of anemia.
- Has a positive effect on the condition of nails, skin and hair.
Helpful information
Find out more about poisonings, their symptoms, treatment regimens, antidotes and consequences:
- Acetylsalicylic acid.
- Carbon monoxide: signs, symptoms, treatment.
- Benzodiazepines.
- Benzodiazepine-like hypnotics.
- Beta blockers.
- Lead, fluorine.
- Calcium antagonist.
- Cardiac glycosides.
- Ethanol.
- Ethylene glycol.
- Histamine
Increased content
Excessive concentration of Fe in the blood, as well as its deficiency, indicates existing pathologies. Since the mechanism for regulating the absorption of this microelement is quite reliable, an increased iron content indicates its breakdown or increased breakdown of red blood cells and the corresponding release of ions of this element.
Causes of elevated Fe levels in the body:
- Hemolytic, folate deficiency, aplastic, B12 anemia, thalassemia;
- Increased absorption in the small intestine when restrictions are violated (hemochromatosis);
- Hemosiderosis due to an overdose of drugs with a high content of Fe, or numerous blood transfusions;
- Lead poisoning, side effects of oral contraceptives, sideroachrestic anemia, which caused a failure of hematopoiesis in the bone marrow;
- Hepatitis of any etiology, liver necrosis, hepatopathy, acute cholecystitis.
When studying test results, it is important to take into account the patient’s possible use of oral iron supplements.
Progress, complications and prognosis
Gradient
The clinic is divided into four stages, which may overlap.
- Often transient symptoms such as lethargy, nausea, vomiting, diarrhea, abdominal pain. Leftover pills cause gray/black vomit and stool. Symptoms disappear after 6-8 hours. When consumed in large quantities, the local effect causes bloody diarrhea.
- Symptom of free interval pg transport and distribution of absorbed iron.
- The acute phase is observed in few patients. It manifests itself in the form of metabolic acidosis, shock, renal failure, and liver necrosis. Possible death within 1-3 days.
- Tension in the gastrointestinal tract due to damage. Complications: hypotension, shock, metabolic acidosis, liver and kidney failure, gastric fibrosis and pyloric obstruction.
Forecast
- The prognosis can be serious, but in most cases the poisoning is not life-threatening.
- Toxic doses for 2-3 years: 400 mg Fe2+.
- Lethal doses: 50 - 300 mg Fe2 + / kg.
- Patients who are alive 72 hours after administration make a full recovery.
Principles of therapy
The primary goal of treatment is to prevent complications. For this:
1. Aspiration and gastric emptying are performed. Vomiting is induced if <1 hour has passed since taking the tablet. This will remove iron from the digestive tract before it is absorbed. Radiologically proven Fe tablets in the stomach are indicative of lavage. Please note that the procedure is contraindicated if the patient is vomiting blood.
2. Patients who are asymptomatic when taken within 6 hours and who show no signs of clinical toxicity do not require treatment.
3. Patients with rare symptoms require only symptomatic treatment.
4. Intravenous hydration for all patients with significant symptoms. Treatment with intravenous fluid to maintain fluid balance is important because hypovolemic shock is a leading cause of premature death from toxicity.
5. Check your blood pressure and heart rate regularly, and control your breathing.
6. The antidote for poisoning is Deferoxamine. This drug increases iron excretion (Fe-binding antidote) and is indicated for severe iron poisoning.
7. If the patient is unaffected after several hours of observation and serum Fe remains low, he is discharged.
Diagnostics
Diagnostic criteria
· History of toxic iron intake.
· Some patients develop symptoms after taking 20 mg Fe/kg body weight.
· Severe poisoning most often occurs after ingestion of > 40 mg Fe/kg body weight.
· S-Fe>90 µmol/l indicates severe poisoning, but it is also worth considering the clinic and the time elapsed after administration.
Patients with symptoms should be treated regardless of the amount consumed.
Disease history
Questions to ask the patient when taking a history:
- How many tablets were taken and for how long?
- What type of preparation?
- How long has it been since you used it?
- Has the patient also taken alcohol, drugs, or other pills?
- Does the patient have any signs of hematemesis or blood in the stool?
- Did you try to induce vomiting? How successful?
When calculating iron intake, it is necessary to take into account the iron content in the tablets (ferrous sulfate 20%, ferrofumarate 33%).
Estimating how many pills a child has taken is often wrong.
Treatment regimen
- Deferoxamine. It binds to Fe and forms a water-soluble compound, ferrioxamine, which is excreted in the urine. She turns pink.
- Deferoxamine is given intravenously in the hospital if severe gastrointestinal symptoms, shock, or coma develop.
- Continuously given equivalent to 15 mg/kg/hour. The dose may be increased to a maximum of 35 mg/kg/hour depending on severity.
Typically, treatment lasts about 24 hours. Side effects may include hypotension and acute respiratory distress syndrome.