Pharmacodynamics and pharmacokinetics
The active ingredient pinaverium bromide is an antispasmodic. Its action is based on two mechanisms: a weak m-anticholinergic effect and selective blocking of calcium channels , which are located on the outer membranes of cells of the digestive tract and urinary tract. The drug reduces tone and eliminates spasms of smooth muscle muscles , reduces the intensity of hydrochloric acid , and improves blood flow to the walls of the gastrointestinal tract.
About 10% of the active substance is absorbed in the intestine. After an hour, the drug reaches its maximum content in the blood plasma. It is important that the drug does not affect the functioning of the heart muscle and blood vessels. The drug is processed in the liver, and pinaverium is excreted in the feces or through the kidneys.
Dicetel®
Manufacturer: ABBOTT HEALTHCARE SAS (France)
tab., cover film-coated, 100 mg: 20 or 30 pcs. Reg. No.: P N014873/01
Clinical and pharmacological group:
Antispasmodic with myotropic and m-anticholinergic effects
Release form, composition and packaging
Film-coated tablets
orange, round, biconvex, with an engraving of “100” on one side and a “∇” icon under the letter “S” on the other.
| 1 tab. | |
| pinaveria bromide | 100 mg |
Excipients:
colloidal silicon dioxide - 2 mg, microcrystalline cellulose - 158.7 mg, talc - 6 mg, magnesium stearate - 3 mg, pregelatinized starch - 34 mg, lactose monohydrate - 36.3 mg.
Film shell composition:
butyl methacrylate copolymer - 16.352 mg, sodium lauryl sulfate - 1.636 mg, talc - 12.946 mg, stearic acid - 2.384 mg, sepispers dry 3203 - 0.682 mg (hypromellose (E464) 55-65%, microcrystalline cellulose (E460) 5-15%, titanium dioxide (E171) 20-30%, sunset yellow dye (aluminum varnish) (E110) 3%).
15 pcs. - blisters (2) - cardboard packs. 20 pcs. - blisters (1) - cardboard packs.
Description of the active components of the drug "Dicetel®"
pharmachologic effect
Antispasmodic drug with a selective effect on the gastrointestinal tract. Inhibits the entry of calcium into intestinal smooth muscle cells. Animal studies have shown that pinaveria bromide reduces the effects of sensory neuron stimulation. Does not have anticholinergic effects. Has no effect on the cardiovascular system.
Indications
- symptomatic treatment of pain, disturbances in the transit of intestinal contents and discomfort associated with functional disorders of the intestine;
- symptomatic treatment of pain associated with functional disorders of the biliary tract;
— preparation for x-ray examination of the gastrointestinal tract using barium sulfate.
Dosage regimen
The drug is intended for oral administration.
The tablets are taken whole during meals, without chewing or dissolving, with a glass of water to prevent contact of pinaverium bromide with the mucous membrane of the esophagus.
Film-coated tablets, 50 mg, are prescribed in a daily dose of 1 tablet. 3 times/day or 2 tablets. 2 times/day. If necessary, the daily dose can be increased to 2 tablets. 3 times/day. In preparation for an X-ray examination of the gastrointestinal tract
- 2 tablets each. 2 times/day for 3 days before the study.
Film-coated tablets, 100 mg, are prescribed in a daily dose of 1 tablet. 2 times/day. If necessary, the daily dose can be increased to 1 tablet. 3 times/day. In preparation for an x-ray examination
- 1 tab. 2 times/day for 3 days before the study.
Side effect
The following adverse events have been reported during post-marketing use. Reports have been spontaneous and there are insufficient data to accurately estimate the incidence of cases.
From the digestive system:
abdominal pain, diarrhea, nausea, vomiting, dysphagia. If the drug is taken incorrectly, damage to the mucous membrane of the esophagus may occur.
From the skin:
rash, itching, urticaria, erythema.
Other:
increased sensitivity.
Contraindications
- lactase deficiency, galactose intolerance, glucose-galactose malabsorption;
- hypersensitivity to the components of the drug.
Due to insufficient data on efficacy and safety, the use of the drug in children under 18 years of age is not recommended.
Pregnancy and lactation
There is insufficient data on the use of pinaverium bromide in pregnant women. The potential risk to humans is unknown. The use of the drug during pregnancy is allowed if the benefit to the mother outweighs the potential risk to the fetus.
In addition, it should be taken into account that the drug contains bromine. In this regard, the administration of pinaverium bromide at the end of pregnancy can cause neurological disorders (decreased blood pressure, sedation) in the newborn.
There is not enough information about the excretion of pinaverium bromide in breast milk. Physicochemical and available data on the pharmacodynamics and toxicology of the drug Dicetel® indicate excretion of the drug in mother's milk, and therefore a risk to the nursing infant cannot be excluded. Dicetel® should not be used during lactation.
special instructions
Due to the risk of damage to the esophageal mucosa, the recommendations for use must be carefully followed. Patients with esophagitis and/or hiatal hernia should pay special attention to the correct use of the drug.
Impact on the ability to drive vehicles and operate machinery
No studies have been conducted on the effect of the drug on the ability to drive a car or use other mechanisms.
Overdose
Symptoms:
overdose can lead to gastrointestinal disorders such as flatulence and diarrhea.
Treatment:
specific antidote is unknown; Symptomatic treatment is recommended.
Drug interactions
Clinical studies have not revealed any interactions of pinaverium bromide with cardiac glycosides, oral hypoglycemic agents, insulin, oral anticoagulants and heparin.
Concomitant use of anticholinergic drugs may enhance the relief of spasms.
There was no effect of the drug on the results of laboratory tests to determine drug concentrations.
Conditions for dispensing from pharmacies
The drug is available with a prescription.
Storage conditions and periods
List B. The drug should be stored in a dry place, protected from light, out of reach of children, at a temperature not exceeding 30°C. Shelf life: 5 years.
Drug interactions
Clinical studies have not revealed any interactions of pinaverium bromide with cardiac glycosides, oral hypoglycemic agents, insulin, oral anticoagulants and heparin.
Concomitant use of anticholinergic drugs may enhance the relief of spasms.
There was no effect of the drug on the results of laboratory tests to determine drug concentrations.
Side effects
After the drug was released on the market, cases of some adverse reactions were reported; it is impossible to reliably determine the frequency of their occurrence.
Possible side effects:
- abdominal pain , nausea and vomiting, diarrhea , dysphagia (reactions occur if dosage recommendations are not followed);
- hypersensitivity reactions to the active substance;
- skin rashes, itching , urticaria and other manifestations of allergies .
Dicetel, instructions for use (Method and dosage)
The tablets are taken during meals , without splitting or chewing .
According to the instructions for use of Dicetel at a dosage of 50 mg, the recommended daily dosage is one tablet once a day or two tablets twice a day. After consultation with a doctor, the dosage can be increased to 300 mg per day.
Three days before examining the intestines with barium, you should take two tablets, three times a day.
For a dosage of 100 mg, the daily dosage is two tablets, in two doses. If necessary, it can be increased to three tablets.
To prepare for an intestinal examination, take one tablet three times a day for three days before the procedure.
The effect of Dicetel on the intensity of abdominal pain in irritable bowel syndrome
Zlatkina A.R., Belousova E.A., Nikitina N.V., Petukhova N.G., Chernogorova M.V.
Irritable bowel syndrome (IBS) occupies a leading place among functional gastrointestinal disorders. In accordance with the international consensus (Rome II criteria), IBS is defined as a complex of functional (i.e. not associated with organic intestinal damage) disorders, the main clinical symptoms of which are abdominal pain and/or abdominal discomfort, with a total duration of at least 12 weeks. over the past 12 months, accompanied by two of three signs: a decrease in symptoms during bowel movements, their connection with a change in the frequency of stool, and a connection with a change in the shape (appearance) of the stool [10,20].
Depending on the nature and combination of clinical symptoms, there are 3 main types of IBS:
- A variant with a predominance of pain and flatulence.
- Option with a predominance of constipation.
- Option with predominance of diarrhea.
With any of these options, the leading symptom of the disease is abdominal pain. The main mechanisms of pain development are due to visceral hyperalgesia and impaired intestinal motility. IBS is characterized by hyperkinetic motor disorders on the part of the circular layer of smooth muscles of the intestinal wall, responsible for intestinal tone, with the development of smooth muscle spasms. In the case of IBS with constipation, smooth muscle spasm is accompanied by impaired intestinal transit [3,20].
The motor function of the intestine is under the control of numerous regulatory influences (central, peripheral, enteric nervous systems and gastrointestinal peptides), which determine the normal tone and contractile activity of the smooth muscles of the intestinal wall [4,7,12,15,23]. At the final stage, the balanced functioning of the smooth muscle apparatus depends on the concentration of calcium ions in the cytoplasm of the myocyte. Calcium ions enter the myocyte through specialized membrane channels [17,22]. The opening of calcium channels leads to an increase in calcium concentration, the formation of the actin-myosin complex and contraction of smooth muscles, and blocking of the channels is accompanied, accordingly, by a decrease in the calcium concentration in the myocyte and its relaxation [17,22].
Since smooth muscle spasm is one of the main components of abdominal pain in IBS, its elimination becomes a very urgent task, but its solution presents significant difficulties due to mixed mechanisms. Treatment of IBS should be comprehensive, aimed at eliminating interdependent central and visceral negative effects [5,6,9,16]. Currently, there are no ideal means for such correction. Myotropic antispasmodics are considered the drugs of choice for relieving spasms of any origin and relieving pain, especially in functional disorders of the gastrointestinal tract (GIT). They affect the final stage of the formation of hyperkinesia, regardless of its cause and mechanism [1,2,5].
It has long been noted that calcium antagonists prescribed in connection with cardiovascular diseases (nifedipine and verapamil) have a relaxing effect on the smooth muscles of the gastrointestinal tract. This was the impetus for the creation of another group of modern effective myotropic antispasmodics - selective blockers of calcium channels in the smooth muscles of the gastrointestinal tract. A classic representative of this group is pinaverium bromide (Dicetel) [17,19].
Clinical trials of the drug "Dicetel" (pinoverium bromide) were carried out on the basis of the gastroenterology department of the Moscow Regional Research Clinical Institute (MONIKI), the therapeutic department of the Central City Hospital in Zhukovsky and the gastroenterology office of polyclinic No. 1 in Podolsk.
Purpose of the study: to evaluate the effect of Dicetel on the intensity of abdominal pain syndrome and dyspeptic symptoms in patients with IBS during a course of treatment; assessment of drug tolerability.
Materials and methods
Characteristics of patients and study design
The study included 30 patients with IBS: 26 women and 4 men with a disease duration of 1-7 years, aged from 21 to 47 years. The diagnosis of IBS was established after a complete clinical examination of the gastrointestinal tract, during which other diseases with similar clinical symptoms were excluded.
The patients were divided into 2 groups. The first group included 13 patients with IBS with a predominance of abdominal pain and flatulence. The second group included 17 IBS patients with constipation.
Patients in both groups received treatment with Dicetel 100 mg (2 tablets) x 3 times a day for 2 weeks as monotherapy. Other drugs that relieve pain, affect the nature of stool or flatulence were not used.
Performance evaluation criteria
To study the clinical effectiveness of Dicetel, the intensity of abdominal pain was assessed in points from 0 to 3 before and after treatment: 0 - no symptom, 1 - mild, 2 - moderate, 3 - severe. Stool disorders were assessed by the frequency of bowel movements per day and consistency. Patients noted the intensity of these symptoms daily in a self-monitoring diary.
The results of using Dicetel were determined as:
- excellent - with complete relief of pain;
- good - reduction in pain intensity by 50-70% (from 2 to 1 point and from 3 to 1 point);
- satisfactory - reduction in pain intensity by less than 50% (from 3 to 2 points);
- without effect - in the absence of positive dynamics or negative dynamics.
Tolerability assessment criteria.
- Good tolerability - no side effects or changes from generally accepted laboratory tests.
- Satisfactory tolerability - the development of mild side effects that do not require discontinuation of the drug, without changes in laboratory tests.
- Poor tolerability - development of moderate or severe side effects, including changes in laboratory parameters requiring discontinuation of the drug.
results
Characteristics of pain syndrome
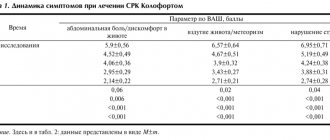
The intensity of abdominal pain in group I patients before treatment with Dicetel corresponded to 2 points in 7 patients (53.8%) and 3 points in 6 patients (46.2%), the average score was 2.46. In group II, similar indicators were noted: 2 points in 8 (47%) and 3 points in 9 (53%) patients, the average score was 2.53. The group of IBS patients as a whole had an average score of 2.5. After two weeks of using Dicetel at the indicated dose, the average score decreased by 62-65% (Table 1). Noteworthy is the fact that the initial level of pain was almost the same in patients with IBS with and without constipation. The degree of reduction in pain intensity in the groups was also the same.
As can be seen from the table, the frequency of achieving a positive effect (excellent, good and satisfactory) was comparable in both groups. Somewhat more often, excellent results were observed in patients of group 1, but the differences were not significant. In both groups, excellent treatment results with complete pain relief were achieved in patients with an initial pain score of 2. With high pain intensity and an initial score of 3, the effect was less pronounced.
Good results with a significant reduction in pain by the end of the second week of treatment were noted in 30% of patients in both groups, and this included patients with both a 2-point and a 3-point initial pain rating. A positive clinical response, including excellent, good and satisfactory results, was achieved in 69.3% of cases in group 1 and in 64.7% in group 2. In total, we observed positive dynamics during treatment in 2/3 of patients (20 people).
In parallel with the decrease in pain, a decrease in flatulence was also noted, but the intensity of this symptom could not be quantified.
Characteristics of the chair
IBS occurred with constipation in 17 patients. The stool patterns of these patients met the diagnostic criteria (Rome II) for IBS with constipation [20]. By the end of the course of treatment, independent, although not regular, bowel movements appeared in 7 patients (41.2%), persistent constipation still remained in 10 patients.
Tolerability assessment
In 29 patients, the tolerability of Dicetel was assessed as good, without the development of side effects. In one case, a significant increase in abdominal pain and flatulence was noted, which required discontinuation of the drug. However, after discontinuation of Dicetel, these phenomena did not stop, and the need arose to prescribe anticholinergics and analgesics. Additional examination at this point did not reveal any organic reasons for the increase in pain, so we believe that the reason for the increase in pain intensity was not the side effects of Dicetel, but the natural course of the disease.
Discussion
The basic drugs for the treatment of any clinical variant of IBS are antispasmodic drugs. To achieve a relaxation effect at the peripheral level, reduce the contractile activity of smooth muscles, eliminate spasms and restore normal transit, gastrointestinal smooth muscle relaxants of two main groups are traditionally used: anticholinergics and myotropic antispasmodics [1,5].
Since the contractile activity of smooth muscles is initiated by the parasympathetic nervous system and stimulation by acetylcholine of M-cholinergic receptors on the cell membrane of the myocyte, the use of anticholinergics is absolutely justified and quite effective [1,5]. However, anticholinergics, having a systemic effect, have a large number of well-known side effects on various organs and systems, which is especially pronounced when taken in a course. This does not allow them to be used for the systematic treatment that IBS patients need.
The group of myotropic antispasmodics includes drugs with different mechanisms of action: paraverine and drotaverine (cAMP phosphodiesterase inhibitors), the selective antispasmodic mebeverine (sodium channel blocker) and selective blockers of calcium channels of gastrointestinal smooth muscles [1,2,3].
The drug Dicetel (pinoverium bromide) belongs to the latter group. The impetus for the creation of these drugs was the observation of the relaxing effect of calcium antagonists, widely used in cardiological practice (nifedipine and verapamil) not only on the vascular wall, but also on the smooth muscles of the gastrointestinal tract. However, these drugs have not found a place in gastroenterology due to their non-selective action and systemic vasodilating and cardiotropic effects.
The advantage of Dicetel, one of the most effective antispasmodics, is based on the selectivity of action in relation to the smooth muscles of the gastrointestinal tract and the absence of systemic anticholinergic effects and systemic vasodilating and cardiotropic effects characteristic of calcium channel blockers of the nifedipine group [13,14,19]. These properties determine the advisability of its use for spastic contractions of the smooth muscles of the stomach and intestines, accompanied by pain in various gastrointestinal disorders, including IBS.
The selectivity of Dicetel's action on the gastrointestinal tract and the lack of effect on the cardiovascular system was proven in a double-blind, placebo-controlled study in 20 patients with various cardiac pathologies. With intravenous administration of the drug, there were no changes in the excitability of the heart muscle, disturbances in sinoatrial or atrioventricular conduction (which can be observed with the administration of drotaverine) and a decrease in blood pressure [13].
At the moment, antispasmodics of direct myotropic action with a selective effect on the smooth muscles of the gastrointestinal tract are an integral and very effective part of the complex treatment of patients with functional gastrointestinal disorders [5].
The purpose of our clinical study was to evaluate the effectiveness and tolerability of Dicetel in relieving abdominal pain in patients with IBS, therefore the drug was used as monotherapy.
In the observed group of patients, what primarily attracted attention was the same intensity of abdominal pain in IBS patients with and without constipation and the same response to the drug in these groups (the same percentage of reduction in pain intensity). It follows that, contrary to expectations, constipation and impaired intestinal transit do not contribute additionally to the development of pain in IBS. This point has not yet been discussed in the literature. The insufficient number of patients in the groups does not allow us to speak with complete certainty about this observation; additional research is required.
The results of the study showed that Dicetel is an effective symptomatic remedy for relieving pain and improving intestinal function in patients with IBS. A positive treatment effect in our patients was obtained in 2/3 of the cases, which we regard as a very good result. It should be noted, however, that the effect of Dicetel with an effectiveness rating of “excellent” in our observations was noted only in a small number of patients. In the vast majority of cases, the results were good and satisfactory. We believe that significant, but not complete, pain relief is due to Dicetel monotherapy, while treatment of IBS requires a comprehensive pathogenetic approach, including the influence on the central and peripheral nervous system, the use of psychotropic and laxatives, and in the future the use of drugs that regulate the functioning of serotonin hormones. receptors [12]. We also attribute the lack of effect in some patients to monotherapy. Our results coincide with earlier studies assessing the effectiveness of Dicetel, in which the frequency of good and very good results in reducing pain intensity in IBS reached 60-64% [14,21].
It is interesting that some patients with IBS with constipation began to have independent bowel movements while taking Dicetel. This was noted in patients with mild to moderate pain. It is possible that the use of laxatives is not always necessary for patients with IBS; restoring intestinal transit with antispasmodics may be sufficient. Similar data were obtained by J. Delmont in a double-blind, randomized study of Dicetel in 60 patients with IBS. A statistically significant disappearance of pain and constipation was noted when taking Dicetel compared to placebo [8]. The effect of Dicetel on the rate of transit through the colon was established in healthy volunteers when taking 50 mg of Dicetel three times a day. These changes were most pronounced in the descending and rectosigmoid colon [11].
The drug was generally well tolerated, except for 1 case (3.3%), when the patient's pain increased while taking the drug. Other studies also noted good tolerability of Dicetel [21]. A frequency of mild side effects similar to ours (4%) was given in the study by B. Noel [18].
In conclusion, it should be said that Dicetel, being a myotropic antispasmodic that selectively affects the tone of smooth muscles of the gastrointestinal tract, can be successfully used in the treatment of abdominal pain in patients with IBS. It is most advisable to include Dicetel in the general complex of treatment measures, but in some cases, with moderate pain, it can be effective as monotherapy.
conclusions
- The intensity of abdominal pain is the same in patients with IBS with and without constipation. Constipation does not appear to contribute additional mechanisms to the development of pain in patients.
- The degree of reduction in pain intensity under the influence of Dicetel in patients with IBS with and without constipation is almost the same.
- Dicetel in the form of monotherapy when used in a course gives a positive clinical effect in more than 60% of patients, regardless of the clinical variant of IBS.
- In some cases, a decrease in pain intensity with Dicetel is accompanied by normalization of stool without additional use of laxatives.
Literature 1. Belousova E.A. Antispasmodics in gastroenterology: comparative characteristics and indications for use - Pharmateka. - 2002, No. 9, pp. 40-46 2. Ivashkin V.T. Metabolic organization of stomach functions. - L., Nauka. - 1981. - 215 p. 3. Poluektova E.A. Abdominal pain with functional intestinal disorders. - Clinical perspectives in gastroenterology and hepatology. - 2001. - No. 2, pp. 27-33 4. Frolkis A.V. Pharmacological regulation of intestinal functions.-L., Nauka.-1981.-204 p. 5. Bulat R., Caldarelli C., Tougas G. Therapeutic utility of antispasmodics in IBS.-In: Irritable bowel syndrome. Ed. by Camilleri M., Spiller R.-WBSaunders, 2002 6. Camilleri M. Review article: clinical evidence to support current therapies of irritable bowel syndrome.- Aliment Pharmacol Ther.- 1999.-v.13, suppl. 2, p.48-53 7. Costa M., Simon JH The Enteric Nervous System.-Amer.J.Gastroenterol.-1994.-v.89, No. 8, p.S129-S137 8. Delmont J. The advantage of adding a musculotropic antispasmodic agent to bran in the treatment of painful constipation in functional bowel syndrome.- Med. Chir.Dig.-1981.- v.10.-p.365-370 9. Drossman DA Review article: an integrated approach to the irritable bowel syndrome.- Aliment. Pharmacol. Ther.- 1999.-v.13, suppl. 2, p.3-14 10. Drossman DA The functional gastrointestinal disorders and the Rome II process.- Gut.- 1999.- v.45, suppl. II, p.1-5 11. Froguel E., Chaussade S., Roche M. et al. Effect of an intestinal smooth muscle calcium channel blocker (pinavrium bromide) on colonic transit time in humans.- J.Gastrointest. Motil.-1990.- v.2 (3).-p. 176-179 12. Gershon MD Review article: roles played by 5-hydroxytryptamine in the physiology of the bowel.- Aliment Pharmacol Ther.- 1999.-v.13, suppl. 2, p.15-30 13. Guerot C., Khemache A., Sebbah J. et al. Electrophysiological study of intravenous pinaverium bromide in cardiology.- Curr. Med. Res. Opin.-1988.-v.11 (2).-p. 73-79 14. Levy CI, Charbonnier A., Cachin M. Pinoverium bromide and irritable colon.- Sem.Hop.Paris Ther. 1977.- v.53 (7-8).-p. 372-374 15. Kamm MA, Lennard-Jones J. Constipation.-Wrightson Biomedical Publishing LTD.- 1994.- 402 p. 16. Malagelada JR Review article: clinical pharmacology models of irritable bowel syndrome.- .- Aliment Pharmacol Ther.- 1999.-v.13, suppl. 2, p.57-64 17. McCallum RW The role of calcium and calcium antagonism in motility disorders of the gastrointestinal tract.- In: Calcium antagonism & Gastrointestinal motility.- Experta Medica.-1989.-p. 28-31 18. Noel B. Estudio multicentrico del bromuro de pinaverio en syndrome de colon irritable llevado a cabo en Mexico (Multicentre study of pinaverium bromide in irritable bowel syndrome completed in Mexico).- Invest. Med. Intern.-1988.-v.15.-p. 190-196 19. Pinoverium bromide (Dicetel).- In: Calcium antagonism & Gastrointestinal motility.- Experta Medica.-1989.-p. 42-45 20. Thompson WG, Longstreth GF, Drossman DA et al. Functional bowel disorders and functional abdominal pain .-Gut.- 1999.- v.45, suppl.II, p.43-47 21. Virat J., Hueber D. Douleur due a la colonopathie et Dicetel. - Prat Med.- 1987.-v.43.-p.32-34 22. Wesdorp ICE. The central role of Ca++ as mediator of gastrointestinal motility.-In: Calcium antagonism & Gastrointestinal motility.- Experta Medica.-1989.-p. 20-27 23. Wood JD, Alpers DH, Andrews PLR Fundamentals of neurogastroenterology.- Gut.- 1999.- v.45, suppl. II, p.6-16
Dicetel's analogs
Level 4 ATC code matches:
Disflatil
Mint tablets
Kuplaton
Espumisan
Espumisan Baby
Espumisan L
Espumisan 40
Bobotik
Dill water
Pepsan-R
Sub Simplex
Infacol
Plantex
Bebinos
Meteospasmil
Romazulan
Common analogues of the drug: Bobotik, Disflatil, Carminativum, Bebinos , Kuplaton Plantex , Platifillin, Romazulan, cumin fruits, fennel fruits, Enterospasmil, Espusin-Zdorovye, Espumisan , Vinboron, Infacol, Kolikid, Duspatalin, Meteospasmil, Sab Simplex, Espicol.
Dicetel or Duspatalin - which is better?
It is believed that Duspatalin has a more pronounced antispasmodic effect, has less pronounced side reactions, and allergies occur much less frequently. However, it is not suitable for emergency treatment with excessive anxiety or, for example, problems with nutrition. In addition, the cost of the analogue is slightly higher than that of the original.
Instructions for use
Dicetel is a myotropic antispasmodic that relieves spasms by relaxing the smooth muscles of the gastrointestinal tract.
The drug simultaneously works in three directions:
1. Relieves spasm by activating the production of calcium and potassium;
2. Does not affect the performance of the cardiovascular system;
3. Reduces the sensitivity of neural endings, reducing pain.
The drug is absorbed into the blood fairly quickly , reaching its highest concentration within an hour after taking the tablet. Almost completely binds to plasma proteins, providing quick results. Metabolized in the liver, after which the metabolites are eliminated to a large extent in the feces.
The advantage of Dicetel is that it can be taken by people with chronic diseases of the cardiovascular system. A muscle relaxant by nature, this drug has an exclusively local effect, relaxing the smooth muscles of the entire gastrointestinal tract, eliminating the risk of lowering blood pressure.
Indications for use
The drug is widely used in gastroenterology for the treatment of the following diseases and pathological processes:
spasmodic pain in the stomach and intestines caused by eating heavy foods (fatty, fried and smoked);- functional disorders of the entire digestive tract;
- spasms of the biliary tract;
- in the treatment of peptic ulcer (as part of complex therapy, as a muscle relaxant drug).
Dicetel is used in preparation for an x-ray examination of all gastrointestinal organs, which requires the administration of barium salts (the drug reduces the reaction of the gastrointestinal tract to barium, eliminating pain).
Mode of application
The tablets are taken during meals with plenty of liquid.
You should not chew or bite them, as the active components can significantly damage the mucous membrane of the esophagus.
Since the drug has different dosages, the number of doses also differs:
- Tablets with an active component of 50 mg are prescribed in several variations: 1 tablet three times a day, or 2 tablets in the morning and evening. The daily dose should not exceed 200 mg, since the likelihood of adverse reactions is high.
- Tablets with an active ingredient of 100 mg - the daily dose is 200 mg (2 tablets), divided into two doses: morning and evening during meals.
It is permissible to increase the daily dosage by the amount determined by the doctor. You should not take the tablets with milk or sparkling water, as this may provoke a reaction during which Dicetel is metabolized earlier than expected, reducing the drug's effectiveness.
In the case where the use of the drug is explained by preparation for an x-ray examination, the patient is asked to start taking Dicetel 3-4 days before the examination date. Prescribe 1 tablet 2 times a day. The maximum permissible daily dose is 300 mg. If it increases on its own, signs of overdose may develop.
If an overdose is diagnosed, the patient experiences symptoms such as:
- stool disorder in the form of prolonged diarrhea;
- feverish state, tachycardia;
- dizziness and loss of consciousness;
- excessive gas formation.
In this case, symptomatic treatment is required, which consists of washing the gastrointestinal tract, as well as taking sorbents. At the time of elimination of the overdose, the drug is completely eliminated.
Pay special attention!!! Under no circumstances should you use tablets with a dosage that does not coincide with the doctor’s recommendations. If you split a 100 mg tablet in half, you will not be able to get an equal dose of 50 mg.
In addition, a damaged membrane and direct contact of the active component with the mucous membrane of the oral cavity and esophagus can provoke bleeding, as well as erosive lesions.
Composition and release form
Dicetel is produced in the form of biconvex tablets, orange in color, with a stripe in the middle. There are two types of dosage: 50 mg and 100 mg. Depending on this indicator, the amount of the active substance changes: pinaverium bromide. Auxiliary components:
- talc;
- silica;
- lactose;
- starch;
- artificial dye.
Tablets are packaged in plastic blisters of 20 pcs. One package contains one blister.
Drug interactions
During the laboratory tests, no adverse reactions from drug interactions were detected. The drug is completely tolerant to any type of medication, without causing pathological changes in the body.
The simultaneous use of Dicetel and anticholinergic drugs is allowed, which increases the effectiveness of the antispasmodic several times.
The drug does not counter-react with other medications, therefore it is approved for use in complex therapy, as well as for people who are forced to constantly take medications (for diabetes, pancreatitis, cholecystitis).
Dicetel price, where to buy
You can buy 20 tablets of 50 mg for 317 rubles.
The price of Dicetel for 100 ml is approximately 485 rubles for 20 pieces.
- Online pharmacies in RussiaRussia
ZdravCity
- Dicetel tablets p.p.o.
100 mg 20 pcs Abbott Healthcare SAS/Mylan Laboratories SAS RUB 723 order - Dicetel tablets p.p.o. 50 mg 20 pcs. Mylan Laboratories SAS
RUR 543 order