Historical information
The history of the antipellagric vitamin is perhaps one of the most fascinating and complex.
Back in 1867, Huber first obtained nicotinic acid by oxidizing nicotine with chromic acid, but only in 1937 was it proven that it is vitamin PP. In 1873 Weidel. obtained nicotinic acid by oxidation of nicotine with nitric acid, and in 1879 by oxidation of beta-picoline. He also suggested its name. At the same time, in 1879, the Russian organic chemist A. N. Vyshnegradsky synthesized nicotinic acid from 3-ethylpyridine. In 1877 Laiblin obtained nicotinic acid by oxidizing nicotine with permanganate. In 1912 Suzuki, Shimamura and Odake isolated nicotinic acid from rice bran, and in 1913, independently of them, Funk isolated it from rice bran and yeast. However, the isolated crystalline substance did not prevent or cure beriberi. In 1926, Vickery again isolated nicotinic acid from yeast. But none of the listed researchers suspected that this substance was a true antipellagric factor. This is even more surprising because around the same time, the American doctor Goldberger identified as the main cause of pellagra a deficiency in human nutrition of a new, hitherto unknown factor PP (pellagra preventing). He tried to make rats deficient in this substance. However, the cause of the disturbances he obtained in the experiment was subsequently found to be vitamin B6 deficiency.
In 1935, V.V. Efremov showed that vitamin B6 does not cure experimental pellagra in dogs.
In 1936, Koehn and Elvehjem found that liver extract did not prevent or cure canine pellagra or pellagra in humans.
In 1936, they obtained an active fraction from liver extract, 64 mg of which cured canine pellagra. From this fraction in 1937, Strong and Woolley obtained a crystalline substance, which turned out to be nicotinic acid.
In 1937, Elvehjem and co-authors established in experiments on dogs in which experimental pellagra was reproduced that nicotinic acid cures this disease. In 1937, nicotinic acid was successfully used for human pellagra.
In 1938, V.V. Efremov, for the first time in the USSR, cured severe pellagra with psychosis with nicotinic acid.
In the course of their search to uncover the etiology of pellagra, Goldberger and Tanner in 1922 hypothesized that the cause of this disease could be a lack of certain amino acids, namely tryptophan, which was later confirmed.
Warburg and Christian in 1934 first showed the importance of nicotinic acid in biochemical reactions. They isolated nicotinic acid amide from codehydrase II (NADP) and established its function as a constituent of the hydrogen transfer coenzyme. Almost simultaneously, in 1935, Euler et al. isolated a substance from codehydrase I (NAD), which was also identified with nicotinic acid amide. The great biological significance of nicotinic acid was then established by a number of studies showing that this substance is an important factor for some microorganisms.
Interaction with other drugs
Vitamin PP enhances the effect of drugs with vasoactive effects, which can lead to a decrease in blood pressure. Concomitant use with bile acid skewestrants is not recommended - they should be used at least 1 hour apart.
Niacin may cause hyperglycemia, so patients should monitor their blood sugar levels. In case of simultaneous use with Amlodipine and Atorvastatino, the risk of developing myopathy may increase. Also, vitamin PP is not recommended to be used together with vitamin C.
It is worth considering that simultaneous use with nicotinic acid reduces the effectiveness of such drugs:
- "Gliquidone";
- "Repaglinide";
- "Insulin Lizpro";
- "Phenobarbital";
- "Metformin";
- "Glipizide".
Chemical and physical properties of vitamin PP
Nicotinic acid is quite easily isolated from most natural products. It is a white, needle-shaped, odorless, crystalline substance with a sour taste and a melting point of 234-237°. Its molecular weight is 123.11. One gram of nicotinic acid is soluble in 60 ml of water and 80 ml of ethyl alcohol at 25°. It is insoluble in ether, but soluble in aqueous solutions of alkali hydroxides and carbonates. Nicotinic acid is not hygroscopic, very stable in dry form. Its solutions can withstand autoclaving at 120° for 20 minutes without destruction. It tolerates boiling well in 1 N. and 2 n. solutions of mineral acids and alkalis. Nicotinic acid has an absorption spectrum in ultraviolet rays with a maximum at 260-260.5 nm. There is a linear relationship between the absorption coefficients of nicotinic acid and its concentration.
According to its chemical structure, nicotinic acid is beta-pyridinecarboxylic or pyridine-3-carboxylic acid. Nicotinamide is a white, odorless, crystalline powder with a bitter-salty taste. It melts at 129-131° and has a molecular weight of 122.12. One gram is dissolved in 1 ml of water and 1.5 ml of 95% ethyl alcohol. It is soluble in acetone, amyl alcohol, ethylene glycol, chloroform, butanol, and slightly soluble in ether and gasoline. Nicotinamide sharply increases the solubility of riboflavin. When dry, it is very stable at temperatures below 50°. In aqueous solution it can be autoclaved at 120° for 20 minutes without visible loss of activity. Under the influence of acids and alkalis, it turns into nicotinic acid.
Nicotinamide has an absorption maximum at 260-261.5 nm. According to its chemical structure, it is an amide of beta-pyridinecarboxylic or pyridine-3-carboxylic acid.
Nicotinic acid can be obtained from nicotine, from beta-picoline, quinoline, pyridine, etc. Nicotinamide can be obtained from nicotinic acid, its esters and from 3-cyano-pyridine. One of the most important analogues of nicotinic acid is 3-acetylpyridine, which is used in animal experiments to reproduce nicotinic acid deficiency, as is another analogue, 6-aminonicotinamide. 3-Acetylpyridine has almost no effect on healthy dogs, since only a small part of it is converted in the body into nicotinic acid, and the majority is excreted in the urine in the form of nicotinate and other compounds. When it was used in experiments on mice at a dose of 3 mg per day, symptoms of nicotinic acid deficiency appeared after 3-4 days.
The toxicity of 3-acetylpyridine LD50 for mice is 300-350 mg/kg, and for rats - 80 mg/kg. The toxicity of 6-aminonicotinamide (LD50 for mice 35 mg/kg) is significantly higher than that of 3-acetylpyridine. At a dose of 2 mg/kg, 50% of the animals died after 11 days.
Isonicotinic acid hydrazide (isonicotinylhydrazide, isoniazid) inhibits the growth of Mycobacterium tuberculosis, which lose about 50% of NAD at a concentration of isoniazid in the medium of 0.1 μg/ml. Based on this, it is successfully used as a treatment for tuberculosis.
Nicotinic acid in medicines
The properties of vitamin B3 have led to its popularity in the pharmaceutical industry. The release form of nicotinic acid has three variations:
- Ampoules. The solution is intended for administering the drug intramuscularly. Injections are prescribed to patients with concomitant gastrointestinal diseases. The advantage of this form is the immediate supply of the substance through the bloodstream into the cells where biochemical reactions occur.
- Capsules. Prescribed for hypovitaminosis, since it is not always possible to replenish the required supply of vitamin with food. Results can be seen a week after starting treatment.
- Pills. They have the same purpose as capsules; they are prescribed to eliminate niacin deficiency.
Storing vitamin PP: keep the sealed preparation in a dry place out of direct sunlight. The shelf life of ampoules is four years, tablets are three years. Before using the substance, check the date of its manufacture and the integrity of the packaging. The authenticity of the drug is confirmed by multi-stage laboratory tests.
Distribution of vitamin PP in nature
Nicotinic acid is quite widespread in plant and especially animal products, which are much richer in nicotinic acid. Of the plant products, the richest are dry brewer's yeast (40 mg%) and baker's press yeast (28 mg%). A significant amount of nicotinic acid is found in grain products. For example, wheat contains over 5 mg%.
The distribution of nicotinic acid in wheat grain is approximately the same as that of thiamine. It is found predominantly in the outer layer of the endosperm, the germ and the bran, with the difference that the bran contains more nicotinic acid and less thiamine than the germ. Wallpaper flour contains all the nicotinic acid, and bread made from it contains 3.5 mg%, in 1st grade flour – 1 mg%, and in bread made from it – 0.7 mg%. Rye is significantly poorer than wheat in terms of vitamin PP - 1.1 mg%. Rye flour contains 1 mg%, and rye bread contains 0.45 mg% nicotinic acid. Corn contains about 2 mg%.
Of the cereals, the richest in nicotinic acid is buckwheat (over 4 mg%), then millet (over 2 mg%), barley (2 mg%), oatmeal (1.6 mg%), pearl barley (1.5 mg%), polished rice (1.6 mg%), semolina - 0.9 mg%.
In corn, as in most other grain crops, 95-98% of nicotinic acid is in a bound form that is not digestible by the body - an ester of a complex structure (niacitin). It is released completely only after alkaline hydrolysis. Nicotinic acid released by alkaline hydrolysis is easily absorbed by the body of animals and humans. Along with this, grain crops such as corn are very poor in tryptophan. This should be taken into account when assessing the content of niacin in diets.
Among other plant products, good sources are legumes, in which nicotinic acid is in digestible form: green peas, lentils, beans, soybeans (2 - 2.5 mg%). A good source of nicotinic acid is coffee beans, which contain from 2 to 10 mg%, depending on the variety and roasting. Peanuts are very rich in nicotinic acid (10 - 16 mg%), then spinach, tomatoes, cabbage, rutabaga, eggplant (0.5 - 0.7 mg%). Potatoes contain 0.9 mg% (boiled 0.5 mg%), carrots - 1 mg%, sweet peppers - 0.9 mg°/0, turnips - 0.8 mg%, red beets - 1.6 mg%, in fresh mushrooms - 6 mg%, in dried mushrooms - up to 60 mg%.
Animal products are very rich in nicotinic acid, with the exception of eggs (0.2 mg%) and milk (about 0.1 mg%). So poultry meat contains 6-8 mg%, lamb -5.8 mg%, beef -4 mg%, veal - over 6 mg%, pork - about 3 mg%, liver - 15-16 mg%, kidney -12 -15 mg%, heart -6 - 8 mg%. Fish is poorer in nicotinic acid than livestock meat. Fresh fish contains on average about 3 mg% nicotinic acid, frozen cod - about 2 mg%, pike - 3.5 mg%, pike perch - 1.8 mg%.
In animal tissues, almost all nicotinic acid is in the form of amide bound to the nucleotides NAD and NADP. In foods of plant origin, the nicotinamide content ranges from 7% (yellow corn) to 70% (potatoes) relative to total niacin. In most products of plant origin, nicotinic acid is distributed mainly in the outer shells. For example, wheat bran contains 330 mcg per 1 g, premium wheat flour - 12 mcg, whole wheat - 70 mcg, polished rice - 0.9 mcg, unpolished rice - 6.9 mcg, rice bran - 96.6 mcg, etc. d.
Nicotinic acid is one of the most stable vitamins in terms of storage and cooking. It is also very resistant to canning processes. In canned food stored for 2 years, its loss does not exceed 15%. There is virtually no loss during freezing or drying. Conventional cooking methods result in losses of 15 to 20% of activity. With some cooking methods, losses reach up to 50%. The composition of the soil can affect the content of niacin in plants. A decrease in the content of major ions in nutrient solutions reduced the content of niacin in oats. Fertilizing the soil with lime or adding nitrates to it increased the content of nicotinic acid in wheat.
Nicotinic acid in the beauty industry
Vitamin B3/PP is regularly used in cosmetology. With the help of drugs you can improve your health, improve your facial skin and hair condition. We suggest considering the popular uses of niacin.
For face
Nicotinic acid is the main element in the nutritional and regenerative process of cells. In addition to its beneficial effect on the functionality of organs, niacin becomes a reliable assistant in the fight for the beauty and health of the skin, which is why it is often used for rejuvenation.
If there is insufficient amount of the substance in the body, the first signs often appear on the skin: red spots, itching, decreased elasticity.
Rated world companies use a 4% solution of nicotinic acid in facial skin care cosmetics. The vitamin is freely available in every pharmacy, so you can add it to your favorite creams yourself.
Positive effects of nicotinic acid on facial skin:
- dilates blood vessels;
- improves blood circulation;
- removes excess fluid from tissues;
- has an anti-inflammatory effect;
- retains moisture necessary for tissues;
- promotes regeneration;
- cleanses the skin and improves its color.
In clinical settings, scientists have discovered another useful property - nicotinic acid reduces the likelihood of skin cancer.
Face mask recipe
An ampoule of nicotinic acid is added to the usual lotion and cream (1 ampoule per 50 g of facial product). The vitamin product is applied as a standard cream and, if necessary, washed off with warm water. It is advisable to mix the drug in a separate small container, since prolonged use of the substance leads to an overdose.
For hair growth
Nicotinic acid is considered a reliable remedy for accelerating hair growth. A small contact of the substance with the skin can provoke an allergic reaction. The allergy in the form of redness will disappear within 20 minutes, leaving no traces. Use the drug on your own with caution.
Sometimes people are skeptical about the properties of nicotinic acid because of the repulsive name. The substance is not associated with smokers; nicotine and nicotinic acid are not the same thing.
Niacin, due to its availability, has become widespread for home use. Women prepare healing masks, shampoos and scrubs based on it. A couple of drops of nicotinic acid are added to your favorite shampoo and your hair is nourished with vitamins during each wash.
The drug has a positive effect on the scalp as it strengthens blood vessels. Nicotinamide instantly penetrates the inner layer of the epidermis, from where it reaches the bulb of each hair. Changes in hair condition can be observed after just five treatments. Folk remedies, despite their effectiveness, at first dry out the hair or enrich it with an unpleasant odor. When hair falls out, a person is ready to endure all the consequences; with “nicotine” you don’t have to worry about such negative accompanying factors.
Vitamin PP is involved in the oxidative processes of the body, so hair has a complex effect. Hair follicles are nourished with vitamins and saturated with oxygen. The main advantage of hair care with nicotinic acid is considered to be hydration along the length and the disappearance of dandruff.
If you are wary of supplements not provided by the manufacturer, take niacin tablets. Thus, you influence hair growth and strengthen the condition of the body.
To get the desired result, it is recommended to undergo a comprehensive course of effects on hair: add niacin in liquid form to shampoo and take the vitamin orally in capsules.
Nicotinic acid is considered a popular drug for hair loss and baldness. Rapid loss of hair is the main signal of problems in the body, so first of all, get examined by a doctor, and under no circumstances start treatment without a prescription.
To stop hair loss, nicotinic acid is rubbed into the scalp onto dry hair. Additional ingredients that the vitamin formula is combined with can improve the effect of the drug:
- decoction of medicinal herbs;
- propolis tincture;
- ginger;
- vitamin E.
The compatibility of the drugs helps to achieve quick results in a short time. The course of treatment lasts for one month, only after consultation with a trichologist. The composition of the product for one rubbing uses only one ampoule of nicotinic acid. The vitamin is applied from the side of the temporal region, using massage movements towards the crown. Before the procedure, draw the liquid into a syringe or pipette, this makes it more convenient to apply the vitamin in even portions to the scalp.
Acid in ampoules must be used immediately after opening, otherwise, when exposed to air, it loses its beneficial properties and becomes unsuitable for further use.
Carefully monitor the body’s reaction to vitamin PP in its pure form; if after the procedure the skin turns red or itches, take an anti-allergenic drug and then dilute the acid with purified water or do not use it at all.
Recipes for hair masks based on nicotinic acid (analogues of expensive cosmetic products):
Egg mask
To make it you will need:
- 1 ampoule of niacin;
- 1 capsule of vitamin E;
- linseed oil – 40 g;
- Eleutherococcus tincture – 20 g.
Directions for use: mix the ingredients and apply to washed, dried hair. After an hour, rinse off the mask and wash your hair under running water.
Mask with aloe and propolis to strengthen hair:
- 40 g nicotinic acid;
- 40 ml aloe juice;
- 40 ml propolis tincture.
Apply the mixture to your hair and rinse after 40 minutes.
After using nicotine-based masks, you will feel as if your scalp is “burning.” This indicates improved blood flow to the hair follicles. When your head itches, this is also a sign of activation of processes. After a 30-day course, a break of at least 4 months is required.
The mechanism of action of niacin allows the vitamin to be used for eyebrows and eyelashes. You can simply dissolve nicotinic acid in ampoules with water and wipe your eyebrows with this liquid in the morning and before bed. An effective remedy for improving the growth of eyelashes and eyebrows is a special mask. To make it you will need:
- castor oil (1 ml);
- burdock oil (1 ml);
- vitamin Aevit (2 capsules);
- nicotinic acid (a couple of drops).
Mix all ingredients and apply overnight to eyebrows and eyelashes. The results will not be long in coming; be careful with the body’s reaction to individual components.
B3 will be useful for men. It is used for beard growth and baldness. Recently, beards and mustaches have become a trend in men's fashion, so men should take care of the subject of their pride. It is better to apply gentle versions of masks to your face so as not to damage sensitive skin. For baldness, vitamin liquid is rubbed into problem areas with massage movements.
Nicotinic acid in the fight against excess weight
Vitamin B3/PP is an important element of metabolism, since nicotinic acid is able to regulate the metabolism of fats and carbohydrates entering the body with food. The burning of calories and a person's weight directly depend on metabolism.
Nicotinamide is predominantly found in protein foods. Another type of vitamin, coenzyme, speeds up metabolism, reducing the effects of bad cholesterol in the body. There is a cleansing of toxins and impurities.
Nicotinic acid, in addition to the listed benefits, plays an important role in the formation of hormonal levels. The substance has detoxifying properties, so it is recommended to include it in the treatment regimen after poisoning or excessive alcohol consumption. The vitamin will be useful for blood vessels and their functionality.
Nicotinic acid has been scientifically proven to help with weight loss. Vitamin PP provokes the body's production of a special substance, serotonin. What is it for? The hormone is responsible for a good mood. Women admit that during times of stress or depression they consume excessive amounts of food, in particular confectionery.
Vitamins should be taken in small doses, gradually increasing the dose by 0.1 g: for the first five days, drink nicotinic acid 0.1 g three times a day, from the sixth to 0.2 g, etc. The daily norm ultimately does not exceed 6 g per day. You should take the vitamin immediately after meals, and never drink it with hot drinks (tea, coffee).
Nicotinic acid displaces vitamin C from the body, so you should additionally drink ascorbic acid in the form of vitamins. How long you need to take the vitamin complex depends on the condition of the body and symptoms.
Niacin is an affordable anti-cellulite remedy. There is a lot of information on the Internet about how to take it. To some extent, the recommendations have a basis for existence, since the substance improves blood circulation. Due to contraindications in the instructions for vitamin B3, it is better to avoid using it unnecessarily. Other methods will help you get rid of cellulite at home: cupping massage, body wraps, contrast showers.
Carefully monitor the dosage of vitamin PP to avoid excess intake of the substance. Before drinking nicotinic acid, consult your doctor and carefully read the instructions for use, pay attention to contraindications.
Methods for determining vitamin PP
The chemical method of determination is based on the use of a reaction with bromine cyanide and then with an aromatic amine. The resulting colored compound is measured photometrically. The reaction proceeds in two stages: obtaining a pyridine derivative by reacting nicotinic acid with bromine cyanide and obtaining a colored dialdehyde compound by reacting with an aromatic amine.
Nicotinic acid is also determined by microbiological methods, most often using the culture of Lactobacillus arabinosus and subsequent turbidimetric determination, as well as with the protozoan Tetrahymena pyroformis. Neither niacin nor nicotinamide fluoresce by themselves, but they can be converted into fluorescent compounds. Such methods are widely used to determine the coenzyme forms of nicotinamide - NAD and NADP. The main metabolic product of nicotinic acid, Nl-methylnicotinamide, is also determined by the fluorimetric method. In various exchange reactions associated with the transfer of hydrogen, pyridine nucleotides, being coenzymes of specific dehydrogenases, act in both oxidized and reduced forms.
In the reduced form, the maximum of the absorption spectrum is in the ultraviolet region at 340 nm. Reduced pyridine nucleotides fluoresce when irradiated with ultraviolet rays. Thus, NADP-H has two absorption spectrum maxima at 260 and 340 nm and one fluorescence spectrum maximum at 457 nm. A parallelism was noted between the presence of fluorescence and the biological activity of the reduced coenzyme.
The most common, rapid, sensitive and simple method for determining nicotinic acid metabolites is the determination of Nl-methylnicotinamide in urine. This method is based on the condensation reaction of Nl-methylnicotinamide with acetone in the presence of alkali with the transition to a fluorescent derivative. In this way, 0.3 μg can be determined in 1 ml of diluted urine. Another metabolite excreted in the urine, 6-pyridone Nl-methylnicotinamide, is also determined fluorometrically.
The content of NAD and NADP in erythrocytes is also determined fluorimetrically, based on the method proposed for their determination in urine. For this purpose, blood proteins are precipitated with trichloroacetic acid. Condensation then occurs with acetone in the presence of an alkali, giving a fluorescent compound that is quantified. The content of NAD and NADP in tissues is also determined.
Deficiency symptoms[edit | edit code]
Nicotinic acid deficiency leads to the development of pellagra. Pellagra mainly affects the skin, gastrointestinal tract and central nervous system. The triad of symptoms - dermatitis, diarrhea and dementia - is often called the three D syndrome. Currently, pellagra is most often observed in alcoholism, protein-energy deficiency and deficiency of many vitamins. Initially, erythema, resembling a sunburn, appears on the back of the hands. Later, other exposed parts of the body (forehead, neck and legs) are affected. The damage can then spread more widely. Pellagra is characterized by symmetrical lesions of the skin, which sometimes darkens, flakes and becomes covered with scars.
Lesions of the gastrointestinal tract are manifested mainly by stomatitis, enteritis and diarrhea. The tongue becomes bright red, swells and sometimes ulcerates. Salivation increases and the salivary glands enlarge. Nausea and vomiting often occur. Sometimes, even in the absence of diarrhea, steatorrhea occurs. Diarrhea, if present, often recurs, and stools may be watery and even bloody.
Damage to the central nervous system is manifested by headache, dizziness, insomnia, depression and memory impairment. In severe cases, delusions, hallucinations, and dementia may occur. Conduction along motor and sensory nerves is disrupted. Laboratory testing usually reveals macrocytic anemia, hypoalbuminemia, and hyperuricemia.
They tried to judge the degree of nicotinic acid deficiency by the excretion of its methylated derivatives (in particular, N-methylnicotinamide) in the urine, but this indicator turned out to be unreliable, as was the level of nicotinamide in the blood or urine. The basis for diagnosis in most cases remains clinical manifestations in combination with the effect of exogenous nicotinamide.
Metabolism of vitamin PP in the body
The fate of nicotinic acid entering the body depends on the type of food and the products it contains. As mentioned above, nicotinic acid, found in a number of grain products in the form of an ester - niacitin, is 95-96% not absorbed by the body of humans, dogs and rats, while niacin, found in animal and legume products, is absorbed by them entirely.
The human, dog and pig body is not able to synthesize nicotinic acid in the quantities necessary to cover the body's need for it, and therefore constantly needs to obtain it from food. Some mammals, such as rats, horses, cows and sheep, can synthesize niacin.
The source of nicotinic acid is tryptophan. Since 1945, a number of works have described the individual stages of the synthesis of nicotinic acid from tryptophan in mammals. There are two ways of endogenous synthesis of niacin in the body of animals: microbial synthesis in the intestines and biosynthesis in tissues. The main transformation of L-tryptophan follows the path of cleavage of its pyrrole ring by tryptophan pyrrolase with the formation of formyl-kynurenine, from which kynurenine and 3-hydroxykynurenine are formed, which are one of the main products of tryptophan dissimilation in the body. 3-hydroxykynurenine is further converted to 3-hydroxyanthranilic acid. After the inclusion of two oxygen atoms, 2-acroleyl-3-aminofumaric acid and quinolinic acid, which is a precursor of nicotinic acid, are formed. As a result of a series of intermediate reactions, nicotinic acid and Nl-methylnicotinamide are formed in omnivores and humans.
With a balanced diet, only a small part of tryptophan is excreted from the body of animals and humans in the urine in the form of specific products of its breakdown. When tryptophan is loaded in the urine, significant quantities of its metabolic products, such as kynurenine, 3-hydroxykynurenine, kynurenic and xanthurenic acids, are released in the urine. The participation of vitamin B6 in tryptophan metabolism in mammals was assumed due to the discovery of xanthurenic acid, one of the products of tryptophan metabolism, in the urine of vitamin B6 deficiency. In addition, a number of authors observed that in case of vitamin B6 deficiency in animals, a decrease in the concentration of NAD and NADP in red blood cells and a decrease in the excretion of Nl-methylnicotinamide in the urine.
It turned out that a derivative of vitamin B6, pyridoxal phosphate, is a coenzyme of kynureninase, involved in the hydrolytic breakdown of kynurenine and 3-hydroxykynurenine. Violation of the kynureninase reaction due to vitamin B6 deficiency leads to disruption of the synthesis of 3-hydroxyanthranilic acid and a decrease in the formation of nicotinic acid.
Nicotinic acid entering the body of humans and omnivores and carnivores is converted to nicotinamide and then methylated to Nl-methylnicotinamide, which is partially oxidized to Nl-methyl-2-pyridone-5-carboxamide. From 40 to 50% of the ingested nicotinic acid is released in this form. In herbivorous animals, nicotinic acid does not transform into amide and is excreted in the urine in free or bound form, and nicotinamide found in the food of these animals is excreted in the form of nicotinic or nicotinuric acids. Methylation of nicotinamide occurs by adding a methyl group to the nitrogen of the pyridine ring. Nl-methylnicotinamide has an adsorption maximum in ultraviolet rays of 264.5 nm. Nl-methylnicotinamide 6-pyridone - 260 and 290 nm.
Calculation of urinary excretion of nicotinic acid metabolites in people receiving various amounts of vitamin PP and tryptophan showed that on average from 55 to 60 mg of tryptophan contained in food is equivalent to 1 mg of nicotinic acid.
Horwitt suggested calling 1 mg of niacin, or 60 mg of tryptophan, "niacin equivalent." Thus, from 1.9 to 5% (on average 3.3%) of tryptophan is converted into nicotinic acid.
Use of niacin in medicine
Due to the peculiarities of its chemical structure, niacin (chemical formula C6H5NO2) is actively used in traditional medicine. Vitamin PP penetrates deeply into the cells of the body, so the range of its use is extremely wide.
Indications for the use of nicotinic acid:
- spinal hernia;
- brain dysfunction (memory impairment, inattention, absent-mindedness);
- depression;
- angina;
- acquired diabetes mellitus;
- alopecia;
- osteoarthritis;
- cardiovascular diseases;
- period;
- multiple sclerosis;
- vitamin deficiency (lack of vitamin B3/PP leads to decreased immunity);
- osteochondrosis, including cervical;
- pellagra – a disease that occurs due to a deficiency of vitamin PP in the human body due to poor nutrition; manifests itself in people suffering from alcoholism and chronic gastrointestinal diseases, but it is also often found in pregnant women;
- dysfunction of the small intestine;
- pathological abnormalities in the functioning of the liver, gall bladder, thyroid gland;
- gastritis;
- dysbacteriosis;
- anorexia;
- malignant tumors;
- headache.
Nicotinic acid will not be superfluous for people who drink alcohol or oral contraceptives; for smokers it is completely irreplaceable. For children, vitamin B3/PP is prescribed exclusively by a pediatrician if there is an urgent need.
Niacin helps fight secondary symptoms of diseases. The drug is used for numbness of the fingers caused by dorsopathies. Used in conjunction with B vitamins.
For prevention, vitamin PP should be taken in tablets; in the treatment of diseases, it is better to use the liquid form of the drug. Treatment regimens and dosages are prescribed after examination and collection of the patient’s medical history.
Participation of vitamin PP in metabolism
Nicotinic acid and nicotinamide are substances necessary for the life of all animal and plant cells. They are part of the coenzymes NAD and NADP and, together with apoenzymes, catalyze the redox reactions of cellular metabolism. This role of nicotinic acid was established even before its value as vitamin PP was discovered. NAD was discovered back in 1905, in 1933 its adenine nucleotide structure was established, and in 1936 NAD in its pure form was isolated from brewer's yeast. It is a white amorphous powder, slightly soluble in phenol and methanol with hydrochloric acid. In ultraviolet rays it has an absorption spectrum of 260 and 340 nm.
NAD is a dinucleotide consisting of nicotinamide, two molecules of ribose, two molecules of phosphoric acid and adenine. NADP has a similar property to NAD to interact with hydrogen and the same absorption spectrum. It contains one molecule of nicotinamide, two molecules of ribose, one molecule of adenine and three molecules of phosphoric acid, differing from NAD by the presence of one phosphoric acid residue at the second position of adenosine.
NAD and NADP are found in all cells of the body of animals and plants. As an example, a table of their content in the tissues of rats is presented.
| NAD+ | NADP+ | |||
| over-n2 | NADP-H2 | |||
| Fabrics | in mmol per | NAD-H in % | in mmol | NADP-H2 in% |
| 1 kg wet weight | per 1 kg wet weight | |||
| Liver | 0,86 | 36 | 0,28 | 97 |
| Heart | 0,72 | 38 | 0,049 | 95 |
| Kidneys | 0,66 | 48 | 0,077 | 95 |
| Diaphragm | 0,65 | 32 | 0,018 | 100 |
| Red blood cells | 0,14 | 40 | 0,011 | 40 |
Food sources[edit | edit code]
Sources of niacin include liver, meat, fish, poultry, nuts and beans, as well as bread and cereals, primarily enriched or coarsely ground. Animal protein is especially rich in tryptophan. Absorption, exchange and excretion. Both nicotinic acid and nicotinamide are easily absorbed in all parts of the intestine and enter all tissues. When therapeutic doses of nicotinic acid or nicotinamide are administered, only small amounts of these substances appear unchanged in the urine, whereas when very large doses are administered, both compounds are excreted in the urine largely unchanged. Nicotinic acid and nicotinamide are converted in the body mainly into N-methylnicotinamide, which undergoes further metabolic transformations. Application. Nicotinic acid, nicotinamide and their derivatives are used for the prevention and treatment of pellagra. Exacerbation of pellagra requires intensive treatment. It is recommended to take these drugs 50 mg orally up to ten times a day. If oral administration is not possible, the drug is administered 25 mg IV 2 times a day or more often. Symptoms of pellagra can appear with two types of metabolic disorders. In Hartnup's disease, tryptophan transport in the intestines and kidneys is impaired. In some cases of carcinoids, large amounts of tryptophan are consumed by tumor cells for the synthesis of 5-hydroxytryptophan and serotonin.
The effect of the introduction of nicotinic acid and its derivatives is observed very quickly. Within 24 hours, severe redness and swelling of the tongue disappear, and steatorrhea decreases. Lesions in the mouth and other mucous membranes heal quickly. Within 24 hours, nausea, vomiting and diarrhea may stop, and discomfort in the epigastrium disappears. abdominal pain and bloating. Appetite improves. Sometimes mental symptoms subside overnight. Confused consciousness clears up, patients calm down, begin to perceive their surroundings and become aware of their condition. Nicotinic acid and its derivatives have such a specific effect in this regard that they can be used as diagnostic agents for overt psychoses in the absence of other signs of pellagra. It is recommended to use large doses of nicotinic acid, especially when psychosis is combined with encephalopathy. Skin lesions, especially those that are weeping, hyperpigmented, and ulcerated, respond more slowly to treatment. The porphyrinuria characteristic of pellagra disappears.
Pellagra can be combined with vitamin B1 deficiency manifested by neuropathy. Vitamin B1 deficiency cannot be treated with nicotinic acid and its derivatives; in such cases, thiamine should be used. The condition of many patients with pellagra is also improved by additional administration of riboflavin and pyridoxine.
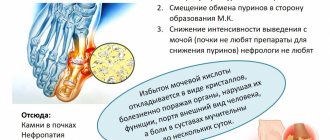
Nicotinic acid in gram doses reduces the concentrations of cholesterol and LDL triglycerides, fibrinogen and lipoprotein(a) in plasma. Therefore, it is used for hyperlipoproteinemia (Chapter 36).
Promising results have been obtained with the use of nicotinamide for the prevention of insulin-dependent diabetes mellitus in at-risk groups (Elliott et al., 1996; Lampeter et al., 1998). Extensive research into this problem is currently underway.
Requirement of humans and animals for vitamin PP
We see that NAD is found in tissues in much larger quantities than NADP. Based on their content in tissues, one can judge the intensity of participation of these coenzymes in metabolism. In cells, the NAD/NAD-H2 ratio is higher than the NADP/NADP-H2 ratio. NAD and NADP in cells, based on the calculation of the enzymatic activity of the entire homogenate, are contained in greater quantities in the nucleus, where their synthesis occurs, and in smaller quantities in mitochondria and microsomes. The enzyme NAD-pyrophosphorylase is part of the enzymes of the cell nucleus, NAD-H -cytochrome C-reductase and NADP-H-cytochrome C-reductase - part of the enzymes of the nuclear membrane itself, NAD-H-dehydrogenase, NAD-H-cytochrome C-reductase, NAD-H-cytochrome B5-reductase, NAD-H- oxidase and NAD- and NADP-isocitrate dehydrogenase - in the composition of mitochondrial enzymes, NAD-H-cytochrome C-reductase, NAD-H2-oxidase, NADP-H2-cytochrome C-reductase - in the composition of enzymes of the endoplasmic reticulum. Thus, NAD and NADP participate as coenzymes in a number of very important enzyme metabolic systems in humans and animals. However, due to the structural features of the protein components of dehydrogenases, the connection of the coenzymes NAD and NADP with these enzymes is less strong than other vitamin-containing enzymes. As a result, NAD and NADP can take part in many oxidation and reduction reactions, migrating from one apoenzyme to another.
The nucleotides NAD and NADP, containing nicotinic acid amide as a catalytically active group, are among the most universal coenzymes in terms of distribution and biological role.
One of the most characteristic physical properties of nicotinamide coenzymes is the presence of reduced forms (NAD-H2 and NADP-H2) of an absorption band in ultraviolet light with a maximum at 340 nm. Excitation of NADP-H2 by radiation with this wavelength leads to the appearance of fluorescence with a maximum at 480 nm.
Spectrophotometric and spectrofluorimetric methods based on these properties are used for the analytical determination of nicotinamide coenzymes, as well as for measuring the activity of associated dehydrogenases.
With the participation of nicotinamide coenzymes, specific dehydrogenases catalyze the reversible reactions of dehydrogenation of alcohols, hydroxy acids and some amino acids into the corresponding aldehydes, ketones and keto acids. Currently, the properties of a large number of enzymes containing nicotinamide as a coenzyme have been isolated and studied.
The most important of these enzymes are:
1. Alcohol dehydrogenases (EC 1.1.1 -2).
R-CH2—OH+NAD (or NADP)—R-CHO + NAD-H (or NADP-H) + H+
2. Aldehyde dehydrogenases (EC 1.2.1.3-5)
R-CHO+H2O+NAD (or NADP)—-R-COOH+NAD-H (or NADP-H) + H+
3. Glucose dehydrogenase (EC 1.1.1.47).
D-glucose + NAD (or NADP) - delta-lactone-D-gluconic acid + NAD-H (or NADP-H) + H+
4. D-glucose-b-phosphate dehydrogenase (EC 1.1.1.49)
D-glucose-b-phosphate + NADP—-delta-lactone-6-phosphate of D-gluconic acid + NADP-H + H+
5. L-glutamic acid dehydrogenase (EC 1.4.1.2-4)
L-glutamic acid + NAD (or NADP) + H2O—— alpha-ketoglutaric acid + NH+ + NAD-H (or NADP-H)
6. L-glycero-3-phosphate dehydrogenase (EC 1.1.1.8)
L-glycero-3-phosphate + NAD - dioxyacetone phosphate + NAD-H + H+
7. Lactic and malic acid dehydrogenase (EC 1.1.1.27-28; 1.1.1.37-40)
R-CHOH—COOH + NADP—— R—CO—COOH + NADP-H + H+
The most important biological function of nicotinamide coenzymes is their participation in the transfer of electrons and hydrogen from oxidizing substrates to oxygen during cellular respiration. NAD and NADP molecules in oxidized form have pronounced acceptor properties, regardless of whether they are obtained by biosynthesis or chemically. It can be concluded that the mechanism of chemical action of these coenzymes is based on the high electron affinity of nicotinamide. Based on quantum mechanics, this is determined by its lowest free molecular orbit. In oxidized forms, NAD and NADP are strong electron acceptors. Since their highest filled orbit is low, they are weak electron donors. For the reduced forms of NAD and NADP, the orbital energies have an inverse relationship, so coenzymes in the oxidized form tend to capture electrons, and in the reduced form, to give them away. We see this in the example of a number of compounds in the formation of which NAD is involved.
Thus, the coenzyme functions of NAD and NADP manifest themselves mainly in redox reactions, in the reversible addition of a hydrogen atom. The main function of coenzymes is expressed in the reversible transformation of the pyridine ring into a 1,4-dihydropyridine ring.
When the pyridine ring is hydrogenated, its light absorption changes. The dihydropyridine system has an absorption maximum at 340 nm, while the pyridine system has almost no absorption in this region. In dehydrogenation processes that are catalyzed by nicotinamide coenzymes, the substrate donates two hydrogen atoms (2H or 2H+ + 2e), but only one H atom joins the coenzyme molecule (in the fourth position of the pyridine ring), and the second H atom gives an electron to the coenzyme and turns into H+ (proton). It has been established that the transfer of the H atom from the substrate to NADP occurs directly and stereospecifically for a given enzyme, always in one direction of the plane of the pyridine core of NADP. Depending on the direction of addition of the hydrogen atom, all dehydrogenases containing NAD are divided into two types - A and B.
Type A includes dehydrogenases of alcohols, L-lactate, L-malate, D-glycerate, acetaldehyde, etc., while type B includes dehydrogenases of L-glutamate, D-glucose, D-glycero-3-phosphate, D-glyceraldehyde -3-phosphate, beta-hydroxysteroids, etc. An example of the step-by-step inclusion of NAD, NAD-H2, NADP and NADP-H2 in the course of enzymatic reactions is the Krebs citric acid cycle. This cycle serves as the crossing point for all important metabolic reactions in which nicotinamide adenine dinucleotides take part.
In some enzymatic reactions, for example in the reaction of anaerobic breakdown of glucose, there are 2 enzymes - lactate dehydrogenase and phosphoglycerin aldehyde dehydrogenase, which are connected by the NAD-NAD-H2 system. This reaction is reversible and its direction is determined by the coefficient NAD/NAD -H2 and the concentration of substances in the reaction.
A special group of enzymes are transhydrogenases, which catalyze reactions between NAD and NADP-H2 towards the dehydrogenation of NADP-H2 at the expense of NAD.
With the help of a specific dehydrogenase, the coenzyme of which is NADP, folic acid is converted into tetrahydrofolic acid (see section “Folic acid”).
A special issue is the structure of the molecule NAD-H, which is a dihydropyridine, having two main types containing an alkyl group at position 1: 1-alkyl-1,2-dihydropyridines and 1-alkyl-1,4-dihydropyridines.
Dihydropyridines containing a urea group in the 3rd position are of greatest biological importance. These compounds have three isomers: 1,2, 1,4 and 1,6.