Composition and release form
The medicine Gaviscon has 2 release forms: tablets and suspension for oral use.
Tablets with a chewable base, slightly flat with a round shape and a light shade. They have minor inclusions over the entire surface, with a pleasant smell of mint or lemon. Packaged in cardboard packaging with 2-4 blisters, each containing 8 pieces. Or in a polypropylene container of 16 tablets with attached instructions for use.
Gaviscon suspension for oral administration has a uniform consistency, light color and menthol flavor. Sold in a dark glass bottle with a volume of 100, 150 or 300 ml.
The active components of the drug are sodium bicarbonate and alginate, calcium carbonate. Their concentration varies depending on the dosage form. Excipients: mint flavor, magnesium stearate, macrogol, aspartame, mannitol, acesulfame potassium.
pharmachologic effect
Gaviscon is a drug for symptomatic treatment. Thanks to the antacid group, it has the property of neutralizing hydrochloric acid in the stomach cavity.
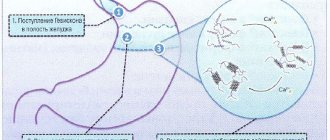
After oral administration, the active ingredients immediately react with the acidic environment. As a result, a gel base with a neutral pH is formed. Due to it, a protective film is formed on the mucous surface of the stomach, preventing gastroesophageal reflux from developing within 4 hours.
In case of severe pathological process, the gel penetrates into the digestive tract, ahead of the entry of gastric contents, where it helps to reduce the irritant factor on the esophageal mucosa.
The therapeutic effect of the drug occurs 3-4 minutes after administration.
The drug does not have general bioavailability. Its pharmacological activity does not extend beyond the gastrointestinal tract. Therefore, the active ingredients rarely penetrate into the bloodstream. This occurs when the therapeutic dose of the drug is increased. Then it is excreted from the body through the kidneys with urine unchanged.
Pharmacodynamics
This drug is a combination of alginate and antacids (calcium carbonate and sodium bicarbonate). When taken orally, the drug quickly reacts with the acidic contents of the stomach. This forms an alginate gel with an almost neutral pH value. The gel forms a protective shell on the surface of the stomach contents and acts for up to 4 hours, effectively preventing the occurrence of gastroesophageal reflux.
In case of regurgitation, the gel enters the esophagus, where it reduces irritation of the mucous membrane. Calcium carbonate quickly neutralizes the hydrochloric acid of gastric juice, relieving the feeling of heartburn. This effect is enhanced by the presence of sodium bicarbonate in the drug, which also has a neutralizing effect.
Instructions for use
According to the instructions, the medication is taken only orally. It is important to observe the therapeutic dose per day and not exceed it.
The tablets are chewed and washed down with a sufficient amount of water. The suspension in powder is kneaded to a homogeneous viscous consistency. As a rule, the course of therapy lasts a week. If there is no pharmacological effect, you should consult your doctor.
Depending on the dosage form, the drug is taken according to the following scheme:
- Tablets - taken after meals and before going to bed, chewing thoroughly. The therapeutic dosage for adults and children over 12 years of age is 2-4 tablets up to 4 times a day. Persons with liver disease and the elderly do not require course correction.
- Suspension – can be used by patients over 6 years of age. Adults are prescribed 10-20 ml, no more than 80 ml per day after meals. For a child 6-12 years old, no more than 40 ml per day, up to 4 times 5-10 ml.
Gaviscon tablets and suspension should be taken with caution if the excretory system is disrupted. Treatment dose adjustment is required depending on the condition.
Gaviscon double action TB chew mints N 12
Release form, composition and packaging
Chewable tablets (mint) are round, flat, two-layer, with beveled edges and a mint odor; one layer has pink color and small inclusions of a darker color, the other layer is white; with an image of a circle and a sword on one side of the tablet and the inscription “GDA250” on the other.
| 1 tab. | |
| sodium alginate | 250 mg |
| sodium bicarbonate | 106.5 mg |
| calcium carbonate | 187.5 mg |
Excipients: mannitol - 598.799 mg, macrogol 20,000 - 30 mg, copovidone - 33.75 mg, acesulfame potassium - 5.863 mg, aspartame - 5.863 mg, azorubine dye (11012) - 0.375 mg, mint flavor - 18.75 mg, xylitol (DC) - 100 mg, magnesium stearate - 6.75-12.6 mg.
pharmachologic effect
Antacid drug. When taken orally, the drug quickly reacts with the acidic contents of the stomach. This forms an alginate gel with an almost neutral pH value. The gel forms a protective barrier on the surface of the stomach contents, preventing the occurrence of gastroesophageal reflux. In the case of regurgitation, the gel is more likely to enter the esophagus, where it reduces irritation of the mucous membrane.
Calcium carbonate quickly neutralizes the hydrochloric acid of gastric juice, relieving the sensation of heartburn and indigestion. This effect is enhanced by the presence of sodium bicarbonate in the drug, which also has a neutralizing effect. The total acid-neutralizing activity of the drug in the minimum dose (2 tablets) is approximately 10 mEq.
Pharmacokinetics
The drug does not have systemic bioavailability (not absorbed).
Indications
- treatment of symptoms of gastroesophageal reflux, such as sour belching, heartburn, dyspepsia, a feeling of heaviness in the stomach that occurs after eating, in patients with high acidity of gastric juice or during pregnancy.
Dosage regimen
The drug is taken orally. The tablet should be chewed thoroughly.
Adults and children over 12 years of age are prescribed 2-4 tablets. after meals and before bed (up to 4 times a day).
The maximum daily dose is 16 tablets.
For elderly patients, no dose adjustment is required.
Side effect
The incidence of adverse reactions was assessed based on the following criteria: very often (≥1/10), often (≥1/100, <1/10), infrequently (≥1/1000, <1/100), rarely (≥1 /10,000, <1/1000), very rare (<1/10,000) and frequency unknown (frequency cannot be calculated from available data).
From the immune system: frequency unknown - anaphylactic and anaphylactoid reactions, hypersensitivity reactions (urticaria).
From the respiratory system: frequency unknown - respiratory effects (bronchospasm).
If any of the side effects indicated in the instructions are aggravated or other side effects not listed in the instructions are noted, the patient should inform the doctor.
Contraindications for use
- moderate to severe renal failure;
- phenylketonuria;
- children under 12 years of age;
- hypersensitivity to any of the components of the drug.
The drug should be used with caution in case of hypercalcemia, nephrocalcinosis and urolithiasis with the formation of oxalate stones, congestive heart failure, and mild renal failure. If you have these diseases or conditions, the patient should consult a doctor before using the drug.
Use during pregnancy and breastfeeding
Clinical studies involving more than 500 pregnant women and the amount of data obtained during the post-registration period did not show feto- and neonatal toxicity of the active substances. Gaviscon® Double Action can be used during pregnancy if clinically necessary and after consultation with a physician.
The duration of use of the drug during pregnancy is no more than 7 days.
Gaviscon® Double Action can be used during breastfeeding.
Use for renal impairment
The use of the drug is contraindicated in moderate to severe renal failure.
The drug should be used with caution in case of nephrocalcinosis and urolithiasis with the formation of oxalate stones, mild renal failure.
Use in children
The use of the drug in children under 12 years of age .
Use in elderly patients
For elderly patients, no dose change is required.
special instructions
In a dose of 2 tablets, the sodium content is 110.75 mg (4.82 mmol). This should be taken into account when a salt-restricted diet is required, for example in congestive heart failure and mild renal failure.
Each dose of 2 tablets contains 150 mg (3.75 mmol) calcium. Therefore, caution should be exercised when treating patients with hypercalcemia, nephrocalcinosis and urolithiasis with the formation of oxalate stones.
Due to the content of aspartame, the drug should not be used in patients with phenylketonuria.
Gaviscon® Double Action contains antacids, which can mask the symptoms of serious gastrointestinal diseases.
The effectiveness of the drug may be reduced in patients with low acidity of gastric juice. In patients with gastroenteritis or suspected renal failure, there is an increased risk of hypernatremia when using the drug.
The drug should not be used for a long time. If symptoms persist after 7 days of using the drug, the patient should consult a doctor to review therapy.
Impact on the ability to drive vehicles and operate machinery
The drug does not affect the ability to drive vehicles and machinery, as well as to engage in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Overdose
Symptoms: bloating.
Treatment: symptomatic therapy.
Drug interactions
The drug contains calcium carbonate, which exhibits antacid activity, therefore, at least 2 hours should elapse between taking Gaviscon®Double Action and other drugs, especially when used simultaneously with histamine H2 receptor blockers, antibiotics from the tetracycline group, digoxin, fluoroquinolones, iron salts, ketoconazole, antipsychotics, levothyroxine sodium, thyroid hormones, penicillamine, beta-blockers (atenolol, metoprolol, propranolol), corticosteroids, chloroquine, bisphosphonates and estramustine.
Storage conditions and periods
The drug should be stored out of the reach of children at a temperature not exceeding 30°C. Shelf life: 2 years.
Conditions for dispensing from pharmacies
The drug is approved for use as a means of OTC
special instructions
The drug contains the chemical element sodium, so it is important for people with pathologies of the heart, kidneys, and when following a therapeutic diet to limit salt to take this into account.
Dose adjustment and strict medical supervision are necessary for nephrocalcinosis, urolithiasis and high calcium levels in the body.
An auxiliary component of the drug is aspartame. It belongs to the group of nonspecific sweeteners, so the medicine can be used for diabetes. On the contrary, it is contraindicated in patients with phenylketonuria.
During clinical trials, Gaviscon did not reveal mutational or teratogenic properties during embryonic development. Pregnant women and nursing mothers can use the drug as prescribed by a specialist.
Since Gaviscon is an antacid, it does not have a direct effect on the nervous system and blood flow. The medicine does not impair memory, does not suppress thinking and concentration, and therefore does not prohibit personal driving.