Pharmacodynamics and pharmacokinetics
After instillation into the eye, it forms a protective film on the cornea, which has moisturizing properties.
The gel can be used to replace the aqueous phase of tear fluid, while it creates an imitation of the mucin layer , creating a moisturizing effect for the conjunctiva and cornea.
Vidisik prevents the formation of epithelial damage and accelerates its regeneration.
This product does not accumulate in body tissues and does not penetrate the eye.
The product can remain on the surface of the eye for no more than 90 minutes.
Pharmacological properties
Pharmacodynamics.
The basis of the drug is a high-molecular hydrophilic polymer, the pH and osmolality of which are similar to those of the normal tear film. Due to its physical properties, the eye gel retains water and forms a transparent and moist film on the surface of the eye. Pharmacokinetics. In pharmacokinetic studies, radioactive carbomer was administered to rats. Research results have shown that only a small amount of carbomer is absorbed. After a single administration, it was found that 0.75% of the dose is excreted in the breath as carbon dioxide and 0.63% is excreted in the urine. The bulk of carbomer (92%) is excreted in the feces within 24 hours after use. Considering the macromolecular nature of carbomer, it can be assumed that it is excreted from the body without undergoing metabolic transformations, that is, it does not go through the hepatic cycle.
The maximum residence time of carbomer on the ocular surface is about 90 minutes.
Vidisik eye gel, instructions for use (Method and dosage)
The medicine must be dripped into each eye, one drop, into the lower conjunctival sac .
The gel can be used three to five times a day. The number of applications per day should be determined based on the severity of complaints and their severity.
The drug should be used thirty minutes before bedtime. Treatment can last for a long period.
People with complaints of dry keratoconjunctivitis use the medicine for a long period; therefore, a specialist should periodically monitor the general condition of the patient, adjusting the dose.
Vidisik eye gel 2mg/g 10g No.1
Name
Vidisik eye gel 2 mg/g in tubes 10 g in pack No. 1
Description
Transparent, colorless, viscous liquid.
Main active ingredient
Carbomer
Release form
Eye gel
Dosage
2 mg
special instructions
Before using Vidisik, you must remove your contact lenses. 15 minutes after Vidisik is instilled, they can be put on again.
Indications for use
Symptomatic treatment of keratoconjunctivitis sicca.
Directions for use and doses
Treatment of dry eyes requires individual dosage selection. Depending on the severity and severity of complaints, instill 1 drop into the eye sac 3 to 5 times a day and 30 minutes before bedtime. Vidisik is suitable for long-term use. When treating keratoconjunctivitis sicca, which is usually long-term or permanent, it is necessary to consult an ophthalmologist. Note: The tip of the tube should not come into contact with the eye or other surfaces, as this may cause injury to the eye and/or lead to microbial contamination of the gel.
Use during pregnancy and lactation
There have been no studies with the drug in pregnant women; on the other hand, there are no doubts that could indicate against the use of the drug during pregnancy and lactation. However, for reasons of principle, it is necessary to use the product during pregnancy and lactation only after carefully weighing the benefits and risks with the help of a doctor.
Precautionary measures
Before using Vidisik, you must remove your contact lenses. 15 minutes after Vidisik is instilled, they can be put on again.
Interaction with other drugs
Unknown. Note: In case of additional treatment with other eye drops, 5 minutes must pass between the application of different medications; in case of additional treatment with eye ointment, wait 15 minutes. In any case, Vidisik is applied last.
Contraindications
Hypersensitivity to any of the components of the eye gel.
Compound
1 g of eye gel contains: Active substance: 2 mg of carbomer (viscosity 40,000-60,000 mPa*s) Other components: Cetrimide, sodium hydroxide, sorbitol, water for injection.
Overdose
No known cases. No special measures are required.
Side effect
To indicate the frequency of side effects, the following criteria are used: Very often (?1/10) Often (?1/100-
Storage conditions
Store at temperatures between 2 and 30 °C. The medicine in unopened packaging is stored for 3 years. After opening the package, the eye gel must be used within 6 weeks.
special instructions
soft contact lenses is not recommended during treatment with the drug . Those who wear contact lenses are advised to remove them before using the eye gel. You can install lenses only fifteen minutes after instillation of the drug.
After each use of the gel, be sure to close the tube. Do not allow the tip of the pipette to touch the surface of the eye. During therapy with this drug, you should not engage in activities that require concentration or speed of psychomotor reactions.
Side effects
Immediately after administration of the drug, temporary blurred vision may occur.
In rare cases, hypersensitivity reactions to some components of the drug and pain in the eyes may occur.
Vidisic contains the preservative cetrimide, which may cause eye irritation, especially with frequent or prolonged use (burning, redness, foreign body sensation in the eye, tingling sensation) and damage to the corneal epithelium. Therefore, for the treatment of chronic forms of keratoconjunctivitis sicca, drugs that do not contain preservatives should be used.
Analogs
Level 4 ATC code matches: Systain Ultra
Systain Gel
Systain Balance
Okoferon
Khilozar-Komod
Hilo-Chest
Visine Pure Tear
Restasis
Catalin
Quinax
Mirtilene Forte
Emoxy optic
Adgelon
Vita-Iodurol
Natural tear
Artificial tear
Optiv
Ophtolic
Balarpan
Taurine
Analogues include the following drugs: Oftagel , Sikapos .
Vidisik
Trade name: Vidisik International name: Carbomer
Release form: eye gel 0.2% (tubes) 10 g
Composition: carbomer 2 mg, excipients (water for injection 957.06 mg, sodium hydroxide 0.84 mg, sorbitol 40 mg, citrimide 0.1 mg) - 1 g
Pharmacological group: keratoprotector
Pharmacological group according to ATK: S01XA20 (Artificial tears and other indifferent drugs)
Pharmacological action: artificial tears, moisturizing the cornea,
Indications: Keratoconjunctivitis sicca and other diseases accompanied by dry eyes.
Dosage regimen: Instill 1 drop into the conjunctival sac 3-5 times a day and before bedtime.
Contraindications: Hypersensitivity.
Side effects: Allergic reactions, transient visual impairment, burning sensation, local eye irritation.
Pharmacodynamics: Ophthalmic agent, gel-like tear substitute. Combined preparation: the base is a hydrogel containing a solid phase (polyacrylic acid) and a liquid external phase (water) as a dispersion medium, polyacrylic acid forms highly viscous, purely crystalline gels, increases the viscosity of the base, sorbitol is used as an isotonic agent, cetrimide is a preservative. Vidisik forms a fluid reservoir on the surface of the eye, moisturizes the conjunctiva and cornea, preventing the formation of epithelial damage or stimulating their healing. The polyacrylic acid skeleton is destroyed by salts contained in the tear fluid, which promotes the release of moisture. Due to the large size of the polyacrylic acid molecule, it does not penetrate intraocularly. Replaces the aqueous phase of tear fluid and imitates the mucin layer.
Pharmacokinetics: Does not penetrate the eye and does not accumulate in tissues.
Special instructions: During treatment, you should not wear soft contact lenses; hard contact lenses should be removed before instilling the drug and reinserted after 15 minutes. During the treatment period, care must be taken when driving vehicles and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions. Carefully. Pregnancy, lactation period.
Interaction: If more than one type of eye drop is prescribed, they should be instilled at intervals of 15 minutes (carbomer should always be instilled last).
Dispensed from pharmacies: Dispensed without a prescription
Drug registration number: P No. 015826/01
Date of registration (re-registration) of the drug: 03/09/2005
Vidisica price, where to buy
The price of Vidisik gel is 240 rubles. Eye drops with similar effects from other manufacturers are sold at similar prices.
- Online pharmacies in RussiaRussia
- Online pharmacies in UkraineUkraine
ZdravCity
- Vidisik ophthalmic gel 0.2% 10gDr.Gerhard Mann, Chem.-Pharm.Fabrik GmbH
RUR 327 order
Pharmacy Dialogue
- Vidisik gel (tube 10g (eye))Dr. Gerhard Mann
RUB 324 order
show more
Pharmacy24
- Vidisic 0.2% 10g gel Dr. Gerhard Mann Chem.-Pharm.
Fabrik GmbH, Nimechchyna 111 UAH.order
Note!
Description of the drug Vidisik eye gel. 0.2% tube 10g on this page is a simplified author’s version of the apteka911 website, created on the basis of the instructions for use.
Before purchasing or using the drug, you should consult your doctor and read the manufacturer's original instructions (attached to each package of the drug). Information about the drug is provided for informational purposes only and should not be used as a guide to self-medication. Only a doctor can decide to prescribe the drug, as well as determine the dose and methods of its use.
Use of Vidisic in the treatment of dry eye syndrome
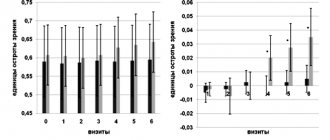
Yu.B. Slonimskiy, GM Chernakova, Yu.M. Koretskaya Vidisik was used for treatment of the dry eye syndrome in 30 patients. Authors describe good results: reduction of corneal syndrome and normalization of tear production. The term “dry eye” has appeared in Russian literature recently. Previously, dry eye syndrome was identified exclusively with Sjogren's disease, which is a severe systemic disease from the group of collagenoses and is accompanied by a decrease or complete absence of secretion of all exocrine glands, especially the lacrimal and salivary glands. It is now generally accepted that dry eye syndrome includes a number of tear film disorders and is a very common disease. The main complaints that a patient has with dry eye syndrome (DES) are redness of the eye, pain, burning, dryness, and foreign body sensation. Typically, such patients show increased sensitivity to cigarette smoke, air-conditioned and cold air, and wind. Damage to the organ of vision in dry eye syndrome leads to persistent visual discomfort, decreased vision due to damage to the corneal tissue and the development of corneal-conjunctival xerosis. Dry eye syndrome can develop after infectious and inflammatory diseases of the eyes, against the background of age-related disorders of tear secretion, against the background of menopause, etc. The new drug Vidisik (manufactured by Bausch & Lomb) is successfully used in the treatment of various forms of dry eye syndrome. The viscous base of the drug is polyacrylic acid, which gives it unique thixotropic properties. This means that under the influence of blinking movements of the eyelids, the drug passes from the gel-like state into the liquid phase, and after a period of rest, the polymer structure is restored again. Thus, the drug remains on the surface of the cornea and conjunctiva for a very long time and restores the structure of the tear film. The composition of the drug Vidisik includes the preservative cetrimide, which does not have toxic properties. At the Department of Ophthalmology with a course in pediatric ophthalmology, a study was conducted of the clinical effectiveness and tolerability of the drug Vidisik in 30 patients with dry eye syndrome of varying severity. The most common complaints were a feeling of dryness in the eye (83%), a feeling of a foreign body (66%), and redness of the eye (60%). More than half of the patients complained of thread-like whitish discharge from the conjunctival cavity. Less commonly reported were photophobia, excessive lacrimation, and sensitivity to tobacco smoke. According to the etiological factor, all patients were divided into 3 groups. Group 1 - 10 people (33%) constantly wore contact lenses. In this group, the predominant manifestation of dry eye syndrome was extensive pinpoint lesions of the corneal epithelium. Corneal syndrome, redness of the eye, and foreign body sensation were noted. Group 2 consisted of 12 people (40%) in whom dry eye syndrome developed after severe bacterial-viral epidemic conjunctivitis. After some time, this group of patients developed sudden periodic redness of the eye and skin of the eyelids, accompanied by mild itching, rapid formation of mucous threads on the surface of the conjunctiva, and visual discomfort. All these phenomena were regarded by ophthalmologists as: 1) relapse of chronic conjunctivitis, 2) allergic reaction of the conjunctiva, 3) chlamydial conjunctivitis. In our opinion, this clinical picture is characteristic of “dry eye” syndrome, which developed after severe epidemic conjunctivitis, accompanied by massive necrosis of goblet cells and decreased tear production. Finally, in 27% of patients, tear production disorders were age-related. In patients over 60 years of age, conjunctival folds parallel to the edge of the lower eyelid, conjunctival scars and cysts, foamy or filamentous discharge from the conjunctival cavity, mucous threads on the cornea, injection of the vessels of the eyeball, and excessive tear production were noted. All patients were prescribed Vidisic as ongoing therapy. The frequency of prescription of Vidisik depended on the severity of the complaints, their persistence, as well as the severity of objective signs of corneal-conjunctival xerosis. In the presence of any complaints that arise periodically, and in the absence of clinically significant lesions of the cornea and conjunctiva, Vidisik eye gel was prescribed once a day. The frequency of use increased as the severity of the clinical picture of dry eye syndrome increased (from 2 or more times a day). During the observation period (from 1 month to six months), we noted relief of corneal syndrome, normalization of tear production, epithelization of defects in the corneal epithelium, which allows us to include the drug Vidisik in the arsenal of drugs for the treatment and prevention of dry eye syndrome.
References 1. G. Hekh. Diagnosis of dry eye syndrome - Moscow 1999. 2. Brzhesky V.V., Somov E.E. Dry eye syndrome: a disease of civilization. Consilium medicum, supplement: Ophthalmology, 2001 3. Maychuk Yu.F., Yani E.V., Maychuk D.Yu. Artificial tear preparation Oftagel in the treatment of dry eye syndrome after epidemic keratoconjunctivitis. Clinical ophthalmology. Volume 2, 2001, No. 4. 4. Heiligenhaus A. et al. Diagnosis and Differentiation in tear Film Deficiencies. Ophthalmologe (1995) 96:6–11, 1995.


![Table 1. Comparison of the results of treatment with Tribestan for men with oligoasthenozoospermia [7] with mod.](https://ms-pi.ru/wp-content/uploads/tablica-1-sravnenie-rezultatov-lecheniya-tribestanom-muzhchin-s-oligoastenozoospermiej-7-330x140.jpg)
