Home | About us | Delivery | Advertisers | Login | Registration
Delivery on Sundays and holidays does not work!
- Medicines
- dietary supplementsVitamins
- Categories from A to Z
- Brands from A to Z
- Products from A to Z
- Medical equipment
- beauty
- Child
- Care
- Honey products appointments
- Herbs and herbal teas
- Medical nutrition
- Journey
- Making medicinesStock
Pharmacy online is the best pharmacy in Almaty, delivering medicines to Almaty. An online pharmacy or online pharmacy provides the following types of services: delivery of medicines, medicines to your home. Online pharmacy Almaty or online pharmacy Almaty delivers medicines to your home, as well as home delivery of medicines in Almaty.
my basket
Apteka84.kz is an online pharmacy that offers its customers medicines, medicinal and decorative cosmetics, dietary supplements, vitamins, baby food, intimate products for adults, medical equipment and thousands of other medical and cosmetic products at low prices. All data presented on the Apteka84.kz website is for informational purposes only and is not a substitute for professional medical care. Apteka84.kz strongly recommends that you carefully read the instructions for use contained in each package of medicines and other products. If you currently have any symptoms of the disease, you should seek help from a doctor. You should always tell your doctor or pharmacist about all the medicines you take. If you feel you need further help, please consult your local pharmacist or contact our GP online or by telephone.
© 2021 Pharmacy 84.
Acyclovir lyophilis d/prig infusion solution 0.25 g fl N1 (Belmedprep)
For adults, for the treatment of infections caused by the Herpes Simplex virus types 1 and 2 with severe immunodeficiency, including after bone marrow transplantation, or impaired absorption from the intestine, it is prescribed intravenously at a dose of 5 mg/kg 3 times a day with an interval of 8 hours. For infections caused by Herpes simplex types 1 and 2 (with the exception of herpetic meningoencephalitis), administer intravenously at a dose of 5 mg/kg 3 times a day with an interval of 8 hours. For herpetic meningoencephalitis, a dose of 10 mg/kg is administered every 8 hours (provided that renal function is not impaired), treatment should begin as soon as possible after the onset of the disease. To prevent infections caused by the Herpes Simplex virus types 1 and 2 in cases of severe immunodeficiency, including after bone marrow transplantation, or impaired absorption from the intestine, it is prescribed intravenously at a dose of 5 mg/kg 3 times a day with an interval of 8 hours. For infections caused by Varicella zoster, administer intravenously at a dose of 5 mg/kg 3 times a day with an interval of 8 hours. For patients with immunodeficiency, the dose is increased to 10 mg/kg every 8 hours (provided that renal function is not impaired). Treatment should begin as soon as possible after the rash appears. To prevent cytomegalovirus infection, the drug is administered intravenously at a dose of 500 mg/m2 3 times a day (every 8 hours), for 5 days before bone marrow transplantation and for 30 days after transplantation. As part of complex therapy for severe immunodeficiency, adults are given a course of intravenous acyclovir therapy for 1 month, and then switch to oral acyclovir. The maximum dose for adults is 30 mg/kg/day. Children aged 3 months to 12 years are usually used at a dose equal to half the adult dose (250 mg/m2 3 times a day, every 8 hours). For the treatment of infections caused by Herpes simplex types 1 and 2, as well as for the prevention of infections caused by the Herpes simplex virus types 1 and 2 in children with immunodeficiency, a dose of 250 mg/m2 is prescribed 3 times a day (every 8 hours). For the treatment of chickenpox in children, use abt at a dose of 250 mg/m2 3 times a day (every 8 hours). For the treatment of chickenpox in children with immunodeficiency and the treatment of herpetic meningoencephalitis, the recommended dose for intravenous administration is 500 mg/m2 3 times a day (every 8 hours), provided that renal function is not impaired. For the prevention of cytomegalovirus infection in children aged 2 years and older, the same doses are recommended as for adults (500 mg/m2 3 times a day, every 8 hours, for 5 days before bone marrow transplantation and for 30 days after transplantation ). Doses of the drug are determined in accordance with the values of creatinine clearance: with a creatinine clearance of 25-50 ml/min, 5-10 mg/kg or 500 mg/m2 are prescribed 2 times a day (every 12 hours), with a creatinine clearance of 10-25 ml/min. min are prescribed at 5-10 mg/kg or 500 mg/m2 1 time per day, with creatinine clearance less than 10 ml/min, prescribed at 2.5-5 mg/kg or 250 mg/m2 1 time per day, subject to hemodialysis or continuous ambulatory peritoneal dialysis. The duration of treatment depends on the patient's condition and response to therapy. The drug is administered intravenously, usually for 5 to 7 days. For herpetic meningoencephalitis, the duration of treatment is usually 10 days. Rules for the preparation and administration of infusion solution for intravenous administration For intravenous administration, the contents of 1 bottle containing 0.25 g are dissolved in 10 ml of sterile water for injection or in a sterile 0.9% sodium chloride solution. The solution can be injected slowly (over an hour) or used dropwise, for which the resulting solution (25 mg in 1 ml) is diluted with an additional 40 ml of solvent (the total volume of the resulting solution is 50 ml, 1 ml contains 5 mg). For intravenous administration, the contents of 1 bottle containing 0.5 g are dissolved in 20 ml of sterile water for injection or in a sterile 0.9% sodium chloride solution. The solution can be injected slowly (over an hour) or used dropwise, for which the resulting solution (25 mg in 1 ml) is diluted with an additional 80 ml of solvent (the total volume of the resulting solution is 100 ml, 1 ml contains 5 mg). For intravenous administration, the contents of 1 bottle containing 1.0 g are dissolved in 40 ml of sterile water for injection or in a sterile 0.9% sodium chloride solution. The solution can be injected slowly (over an hour) or used dropwise, for which the resulting solution (25 mg in 1 ml) is diluted in an additional 160 ml of solvent (the total volume of the resulting solution is 200 ml, 1 ml contains 5 mg). It is necessary to achieve complete dissolution of the drug crystals. Freshly prepared solutions should be used. For adults, when using acyclovir in doses up to 500 mg, the drug must be administered in a liquid volume of at least 100 ml. If it is necessary to administer the drug in high doses (from 500 mg to 1000 mg), the volume of injected fluid should be increased. Intravenous administration is carried out slowly, drip-wise, over 1 hour, regardless of the dose of the drug. The drug can be administered using an injection pump with adjustable flow. The solution prepared for intravenous administration should be stored at a temperature of 15-25°C for no more than 12 hours. In case of turbidity, crystallization during dilution, administration or storage, the use of the solution is excluded.
Acyclovir
Dosage form
Cream for external use
Compound
100 g contains:
Active substance
: acyclovir – 5.0 g.
Excipients
: propylene glycol – 8.0 g, petroleum jelly (liquid paraffin) – 12.0 g, cetyl alcohol – 6.0 g, macrogol 6 cetostearyl ether – 1.5 g, macrogol 25 cetostearyl ether – 1.5 g, methyl parahydroxybenzoate – 0.15 g, propyl parahydroxybenzoate – 0.05 g, water – up to 100.0 g.
Description
A homogeneous cream of white or almost white color.
Pharmacotherapeutic group
Antiviral agent.
ATX code
: D06BB03.
Pharmacological properties
Pharmacodynamics
Acyclovir is an antiviral drug that is highly active in vitro against Herpes simplex virus (HSV) types 1 and 2. After entering cells infected with the herpes virus, acyclovir is phosphorylated to the active compound acyclovir triphosphate. The first step of this process depends on the presence of the HSV-encoded thymidine kinase enzyme. Acyclovir triphosphate acts as an inhibitor and substrate of herpes-specific DNA polymerase, preventing further synthesis of viral DNA, without affecting normal cellular processes.
Pharmacokinetics
Pharmacological studies have shown minimal systemic absorption with repeated use of acyclovir cream.
Pharmacokinetics in special groups of patients
Patients with impaired renal function
In patients with chronic renal failure, the plasma concentration is up to 0.78 mcg/ml.
Indications for use
Treatment of viral infections caused by the Herpes simplex virus, lips and facial skin (recurrent herpes of the lips).
Contraindications
Hypersensitivity to acyclovir, valacyclovir, propylene glycol or other components of the drug.
Use during pregnancy and breastfeeding
Pregnancy
During pregnancy and breastfeeding, it is used only if the expected benefit to the mother outweighs the potential risk to the fetus and child. As a result of post-registration experience with the use of acyclovir, data were obtained containing information on the results of pregnancy in women receiving acyclovir in any dosage form. There was no increase in the number of congenital malformations or any defects among patients treated with acyclovir compared with the general population. Before use, if you are pregnant, or think you might be pregnant, or are planning a pregnancy, as well as during breastfeeding, you should consult your doctor.
Breastfeeding period
Data when using the drug during breastfeeding are limited. With regular use, acyclovir passes into breast milk, but the dosage that a breastfed infant can receive is negligible.
Directions for use and doses
Externally.
Adults and children: it is recommended to apply the drug in a thin layer to the affected and adjacent areas of the skin 5 times a day, approximately every 4 hours, with the exception of night time. It is important to start treatment as early as possible, preferably when the first signs and symptoms appear (in the prodromal period or with redness). Treatment can also begin at later stages (papule or blister). The duration of treatment is at least 4 days. If there is no healing, treatment can be continued for up to 10 days. If symptoms persist for more than 10 days, you should consult a doctor. To prevent the spread of infection, you must wash your hands before and after applying the drug, do not rub or touch the affected areas of the skin with a towel.
Use the drug only according to the method of use and in the doses indicated in the instructions for use. If necessary, please consult your doctor before using the medicine.
Side effect
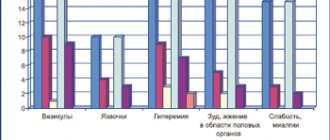
Side effects are classified by organ system and frequency. The frequency of side effects is defined as follows: very often > 1/10, often > 1/100 and < 1/10, uncommon > 1/1000 and < 1/100, rarely > 1/10000 and < 1/1000 and very rarely < 1/10000.
From the skin and subcutaneous tissues
Uncommon – short-term burning, tingling in areas where the drug was applied, slight dryness or flaking, itching.
Rarely - redness, contact dermatitis after application, more often associated with a reaction to excipients than to acyclovir.
Immune system disorders
Very rarely - immediate hypersensitivity reactions, including angioedema, urticaria.
If you experience the side effects listed in the instructions, or they get worse, or you notice any other side effects not listed in the instructions, tell your doctor.
Overdose
When using the drug in accordance with the instructions for use, an overdose is unlikely, due to the minimal absorption of acyclovir. If you accidentally ingest the drug or suspect an overdose, consult a doctor.
Interaction with other drugs
When used externally, no interactions with other drugs have been identified.
special instructions
Acyclovir, a cream for external use, should be used exclusively for herpes of the skin of the lips and face. It is not recommended to use on the mucous membranes of the mouth, eyes and vagina. Do not use for the treatment of genital herpes. Avoid getting the cream into your eyes.
In case of severe manifestations of recurrent herpes, it is recommended to consult a doctor.
Patients with herpes labialis should avoid transmitting the virus, especially if active lesions are present.
Patients with immunodeficiency conditions are not recommended to use acyclovir cream for external use. Such patients should follow the doctor's recommendations when treating any infectious diseases.
Patients with renal and liver failure
Despite the fact that acyclovir is mainly excreted by the kidneys, systematic absorption of acyclovir after external use is negligible. Accordingly, there is no need to change the dose in patients with renal or hepatic impairment.
The effect of the drug on the ability to drive vehicles and machinery
The drug does not affect the ability to drive or engage in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Release form
Cream for external use 5%.
5 g, 10 g, 15 g, 20 g, 25 g, 30 g, 35 g, 40 g, 50 g, 60 g, 70 g, 80 g, 90 g, 100 g in orange glass jars with a triangular rim with tensioned lid with a sealing element.
5 g, 10 g, 15 g, 20 g, 25 g, 30 g, 35 g, 40 g, 50 g, 60 g, 70 g, 80 g, 90 g, 100 g in polymer jars complete with lids or in polyethylene terephthalate jars with closures.
1 g, 2 g, 3 g, 4 g, 5 g, 10 g, 15 g, 20 g, 25 g, 30 g, 40 g, 50 g, 60 g, 70 g, 80 g, 90 g, 100 each g in aluminum tubes coated with BF-2 varnish, with caps made of high-density polyethylene or in polymer tubes with polyethylene screw caps.
Each jar and tube, along with instructions for use, is placed in a cardboard pack.
Storage conditions
At a temperature not higher than 25 °C.
Keep out of the reach of children.
Best before date
2 years.
Do not use after the expiration date stated on the packaging.
Vacation conditions
Available without a prescription.
Acyclovir, 1 piece, 0.25 g, lyophilisate for solution for infusion
The drug is used only in a hospital setting.
Adults
In obese patients, dosages are recommended as in adults with normal body weight.
Treatment of infections caused by herpes simplex viruses (HSV; excluding herpes encephalitis) and varicella zoster virus (VZV)
Intravenous infusions at a dose of 5 mg/kg body weight every 8 hours.
Treatment and prevention of infections caused by HSV, VZV, and herpetic encephalitis in patients with immunodeficiency
Intravenous infusions at a dose of 10 mg/kg body weight every 8 hours with normal renal function.
Prevention of CMV infection during bone marrow transplantation
500 mg/m2 of body surface intravenously 3 times a day with an interval of 8 hours.
The duration of treatment is from 5 days before transplantation to 30 days after transplantation.
Special patient groups
Children
Doses of the drug
Acyclovir in children aged 3 months to 12 years is calculated depending on body surface area.
In newborns, doses are calculated depending on body weight. For infections caused by HSV, a dose of 10 mg/kg every 8 hours is recommended.
Treatment of infections caused by HSV (except herpetic encephalitis) and VZV
Intravenous infusions at a dose of 250 mg/m2 every 8 hours.
Treatment of herpetic encephalitis and VZV infections in children with immunodeficiency
Intravenous infusions at a dose of 500 mg/m2 every 8 hours with normal renal function.
Prevention of CMV infection in children over 2 years of age
Limited data suggest that children over 2 years of age who have undergone bone marrow transplantation may be given an adult dose of Acyclovir.
In children with reduced renal function, dose adjustment is required according to the degree of renal failure.
| Creatinine clearance (ml/min/1.73 m3) | % of recommended dose | Multiplicity introduction |
| >50 | 100 | 4 times a day |
| 25-50 | 100 | 2 times a day |
| 10-25 | 100 | 1 time per day |
| 0-10 | 50 | 1 time per day |
Elderly patients
In the elderly, the clearance of acyclovir in the body decreases in parallel with a decrease in creatinine clearance. In elderly patients with reduced creatinine clearance, a dose reduction should be considered.
Patients with kidney failure
Intravenous infusions of Acyclovir should be administered with caution in patients with renal failure. The following dose adjustment scheme has been proposed depending on the degree of decrease in creatinine clearance:
| Creatinine clearance | Doses |
| 25-50 ml/min | 5-10 mg/ml every 12 hours |
| 10-25 ml/min | 5-10 mg/ml every 24 hours |
| 0 (anuria) - 10 ml/min | For continuous ambulatory peritoneal dialysis, 5-10 mg/ml every 12 hours. For hemodialysis, 5-10 mg/ml every 24 hours and after dialysis. |
The course of treatment with Acyclovir in the form of intravenous infusion is usually 5 days, but may vary depending on the patient's condition and response to therapy. The duration of treatment for herpetic encephalitis and HSV infections in newborns is usually 10 days.
The duration of prophylactic use of the drug Acyclovir for intravenous infusion is determined by the duration of the period when there is a risk of infection.
Preparation of solution and method of administration
The recommended dose of Acyclovir should be administered as a slow intravenous infusion over 1 hour.
Use 10 ml of dilution solution (water for injection or sodium chloride solution for injection (0.9%)) to prepare a solution of the drug Acyclovir containing 25 mg of acyclovir in 1 ml of the resulting solution. The recommended volume of dilution solution must be added to the bottle with Acyclovir powder and shaken carefully until the contents of the bottle are completely dissolved.
After dilution, the Acyclovir drug solution can be administered as an intravenous infusion using a special infusion pump that regulates the rate of drug administration.
Another method of infusion administration is possible, when the prepared solution is diluted further to obtain an acyclovir concentration not exceeding 5 mg/ml (0.5%).
To do this, you need to add the prepared solution to the selected infusion solution, which is recommended below, and shake well until the solutions are completely mixed.
For children and newborns who need to administer minimal volumes of infusion, it is recommended to add 4 ml of the prepared solution of the drug Acyclovir (100 mg of acyclovir) to 20 ml of solvent.
For adults, it is recommended to use infusion solutions in 100 ml packs, even if this will give an acyclovir concentration significantly lower than 0.5%. Thus, one 100 ml infusion solution can be used for any dose of acyclovir between 250 mg and 500 mg (10 and 20 ml diluted solution). For doses between 500 and 1000 mg of acyclovir, another infusion solution of this volume (100 ml) should be used.
Acyclovir is compatible with the following infusion solutions and, when diluted with them, remains stable for 12 hours at room temperature (15 to 25 ° C):
— Sodium chloride for intravenous infusion (0.45% and 0.9%);
— Sodium chloride (0.18%) and dextrose (4%) for intravenous infusion;
— Sodium chloride (0.45%) and dextrose (2.5%) for intravenous infusion;
- Hartmann's solution.
When preparing Acyclovir solution for infusion, as indicated above, the concentration of Acyclovir is no more than 0.5%.
Dissolution and dilution must be carried out completely under aseptic conditions immediately before administration of the drug. Unused solution is discarded. If the solution becomes cloudy or crystals fall out, it should be destroyed.