Interference:
- Beta blockers (rare), amiloride, aminocaproic acid, ACE inhibitors, cyclophosphamide, vincristine, arginine, cephaloridine, cyclosporine, digoxin, adrenaline (initial effect), foscarnet sodium, heparin, histamine (iv), isoniazid, lithium, mannitol.
- Beta-adrenergic agonists (salbutamol, terbutaline), albuterol, aminoglycosides, p-aminosalicylate, aminosalicylic acid (rare), amphotericin, azlocillin, bisacodyl, capreomycin, carbenicillin, carbenoxolone, cholestyramine, cisplatin, clopamide, corticosteroids, corticotropin, cyanocobal amine, dextrose anhydride, EDTA, diclofenamide, diuretics, enoxolone, fluconazole, glucagon, glucose, ifosfamide, insulin, levodopa, licorice, mezlocillin, nafcillin, penicillin G (sodium salt), phenolphthalein, piperacillin, polymyxin B, salicylates, sodium bicarbonate, sodium chloride, ticarzi llyn, theophylline.
Potassium test
04/06/2021
Potassium
What is the role of potassium in our body?
Potassium plays an important role in the processes of muscle contraction, heart function, and conduction of nerve impulses.
What foods are richest in potassium?
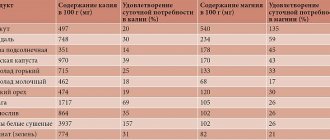
- Dried apricots (100 g of dried apricots contain 1.7 g of potassium)
- Beans (1.1 g per 100 g of product)
- Sea kale (0.97 g per 100 g of product)
- Parsley (0.8 g per 100 g of product)
- Potatoes (0.57 g per 100 g of product)
The average daily requirement for potassium is about 3 g.
People suffering from chronic renal failure or arrhythmia need to limit potassium intake (as recommended by their doctor)
When is it necessary to take a potassium test?
- Preventive examination (comprehensive assessment of electrolyte balance)
- Cardiovascular diseases (hypertension, coronary heart disease, arrhythmias)
- Kidney diseases (chronic kidney disease, urolithiasis, glomerulonephritis)
- Monitoring therapy with diuretics and potassium-containing drugs
- If you have symptoms of hyperkalemia (high potassium in the blood): excitability, diarrhea, convulsions, oliguria, cardiac arrhythmia
- If you have symptoms of hypokalemia (low potassium in the blood): malaise, thirst, polyuria, anorexia, weak pulse, low blood pressure, vomiting)
Why might blood potassium levels be elevated?
- Excessive intake of potassium into the body (food, dietary supplements, medications)
- Redistribution of potassium in the body (convulsions, massive tissue damage, prolonged fasting)
...why is it reduced?
- Reduced intake of potassium into the body (insufficient intake of potassium from food)
- Potassium loss from the body (vomiting, diarrhea)
How do medications affect blood potassium levels?
- Some medications increase potassium levels: nonsteroidal anti-inflammatory drugs, beta blockers (bisoprolol), ACE inhibitors (captopril, enalapril, lisinopril), potassium-sparing diuretics (amiloride, triamterene, spironolactone), heparin.
- The following can cause a decrease in potassium concentration in the blood: diuretics, glucocorticosteroids, beta-agonists (isoprenaline), alpha-agonists (clonidine), antibiotics (gentamicin)
Sodium
What is the role of sodium in our body?
Sodium is involved in maintaining osmotic pressure and regulating the acid-base state, neuromuscular excitability, and transmission of nerve impulses.
What foods are richest in sodium?
- Medium salted herring (4.8 g sodium per 100 g product)
- Pollock roe (2.2 g per 100 g)
- Chicken egg (0.134 per 100 g)
- Sauces, mayonnaise, sausages, cheeses are rich in sodium
The average daily sodium requirement is about 1.3 g.
People suffering from kidney disease, arterial hypertension, gout, and liver disease should limit their potassium intake (as recommended by their doctor).
When should you get a sodium test?
- Kidney diseases
- Excessive fluid loss
- Therapy with diuretics
- Electrolyte balance assessment
Why might blood sodium levels be elevated?
- Fluid loss due to vomiting and diarrhea
- Insufficient fluid intake into the body
- Excessive consumption of table salt
Why might blood sodium levels be low?
- Insufficient sodium intake in the body
- Acute renal failure
- Decreased functional activity of the thyroid gland (hypothyroidism syndrome)
- Overdose of diuretics (diuretics)
What medications can affect blood sodium levels?
Many medications affect sodium levels.
They increase it - corticosteroids, calcium, estrogens, androgens, laxatives, oral contraceptives, and decrease it - heparin, diuretics, anticonvulsants (carbamazepine), tricyclic antidepressants.
Calcium
What is the role of calcium in our body?
Calcium is necessary for the contraction of skeletal muscles and the heart, for the transmission of nerve impulses, as well as for normal blood clotting, and for building the frame of bones and teeth.
About 99% of calcium is concentrated in the bones and only less than 1% circulates in the blood. Almost half of the calcium in the blood is metabolically active (ionized calcium), the remainder is bound to proteins and ions and is inactive.
The average daily requirement for calcium is about 1.2 g (except for categories of people with an increased need for calcium).
Dairy products (cottage cheese, kefir, milk, cheeses) are primarily rich in calcium.
Total calcium in the blood is the total level of its ionized and bound forms.
Only ionized calcium can be used by the body. When is it necessary to take a calcium test?
Disorders of calcium metabolism in various diseases (chronic renal failure, diseases of the thyroid and parathyroid glands, gastritis)
Why can the level of calcium in the blood be increased?
- Hyperparathyroidism is a disease of the parathyroid glands
- Oncological diseases
- Excessive consumption of dietary supplements containing calcium
Why might blood calcium levels be low?
- Chronic renal failure
- Atrophic gastritisHypoparathyroidism – disease of the parathyroid glands
- Insufficient ka intake (in children of puberty, in people with increased physical activity, in pregnant and lactating women, in people over 60 years old)
What medications can affect blood calcium levels?
In some people, calcium levels are elevated by certain medications: androgens, thiazide diuretics (the most common cause), lithium salts, progesterone, parathyroid hormone, tamoxifen, vitamins D and A.
Other drugs, on the contrary, can cause a decrease in calcium concentration in the blood: gentamicin, calcitonin, anticonvulsants (carbamazepine), glucocorticoids, laxatives, magnesium salts.
The concentration of calcium in the blood and urine does not indicate the total calcium content in the bones - for this there is a special technique for determining bone mineral density, called densitometry.
Magnesium
What is the role of magnesium in our body?
Magnesium is a macronutrient necessary for the functioning of the nervous system, muscle contractions, growth and strengthening of bone tissue.
Magnesium is called the “element of calm” because this mineral is extremely important for brain function. The average daily requirement of an adult for magnesium is about 400 mg.
When is it necessary to take a magnesium test?
- If you have symptoms of nervous system exhaustion (fatigue, weakness, irritability, insomnia)
- Neurological pathology (convulsions, tremors)
- Palpitations not associated with heart or thyroid disease
Why might magnesium levels in the blood be elevated?
- Kidney diseases
- Dehydration
- Overdose of magnesium drug
Why might blood magnesium levels be low?
- Lack of magnesium in the diet
- Pancreatic diseases
- 2nd and 3rd trimester of pregnancy
- Excessive physical and emotional stress
What medications can affect magnesium levels in the blood?
Diuretics, immunosuppressants and cytostatics can increase the level of magnesium in the blood.
Test results are not a diagnosis!
The interpretation of the results is carried out by the doctor, taking into account the patient’s symptoms and complaints, medical history, and the results of additional laboratory and instrumental studies.
Dialine Laboratory offers the following tests:
- Potassium (KB16) Sodium (KB28)
- Magnesium (KB24)
- Calcium (KB17)
- Ionized calcium (KB37)
- Urine calcium (CB49).
Select an address for testing.
Any questions?
Fill out the feedback form, our managers will contact you!
Ask a Question
Interpretation:
- Rapid infusion of potassium solution, massive hemolysis, severe tissue damage, acute starvation, hyperkinetic activity (epilepsy), malignant hyperpyrexia after anesthesia, acidosis, dehydration, acute renal failure, final stage of chronic renal failure, Addison's disease, hypofunction of the renin-angiotensin-aldosterone system , pseudohypoaldosteronism, other sodium-wasting conditions, after strenuous exercise (especially in people taking beta blockers), shock, severe hemolysis, tissue ischemia.
- Chronic fasting, vomiting, diarrhea, loss through intestinal fistula, intestinal villous adenoma, renal tubular acidosis, renal tubular failure, Fanconi syndrome, primary and secondary aldosteronism, Cushing's syndrome, Bartter's syndrome, osmotic diuresis (eg, with hyperglycemia), alkalosis, with diabetic ketosis during the period of gluconeogenesis; with the administration of ACTH, cortisone or testosterone, cystic fibrosis.
Sample result (PDF)
Potassium and its significance in a biochemical blood test (K)
Potassium (“K”) is an essential chemical element that creates the necessary conditions for various reactions to occur. It is found in the body in the form of cations - positively charged ions located inside the cell (in the intracellular fluid).
The content in the intercellular fluid is no more than 2% - this is exactly the potassium that is determined during biochemical analysis. A change in this indicator can be observed both in various physiological conditions and in pathology, and a decrease in the concentration in the blood - hypokalemia - is life-threatening and can have serious consequences.
The norm is 3.4 – 5.0 mmol/l. At the same time, it provides:
- Correct heart rate;
- Myocardial contraction;
- The work of skeletal muscles;
- Conduction of nerve impulses through neurons;
- Water-salt balance.
For normal maintenance of the above functions, a sufficient supply of “K” from food is necessary, since there are no own reserves of this chemical element. A lot is found in vegetables (especially greens, potatoes), fruits and grains.
The level of “K” is affected by the rate of its elimination. A decrease in concentration is caused by:
- Kidney diseases;
- Water-salt and acid-base imbalance;
- Hormonal changes (especially mineralocorticoids);
- Uncontrolled use of diuretics;
- Intoxication with repeated vomiting or diarrhea;
- Severe inflammatory processes;
- Deficiency of other minerals;
- Disorders of respiratory and circulatory function.
Hypokalemia – level in biochemistry less than 3.5 mmol/l. At the same time, it develops:
- Severe fatigue;
- Muscle pain;
- Arrhythmia up to cardiac arrest;
- Reduced activity of gastrointestinal motility with subsequent constipation up to dynamic intestinal obstruction;
- Slowing reflexes;
- Slowing down of mental processes;
- Pathology of urination.
Conditions in which the norm is exceeded - hyperkalemia - are somewhat less common. However, this happens when:
- Kidney failure;
- Reverse mineralocorticoid disorders;
- Release of the cation into the intercellular space due to extensive injuries, tumors, poisoning, taking antibiotics or chemotherapy drugs, acid-base imbalance;
- Overdose of drugs with “K”;
- Enhanced diet.
The clinical picture of hyperkalemia is similar to the symptoms that occur with ion deficiency. This is due to the general nature of the occurrence of such signs - a violation of the electrochemical potential of membranes, which is characterized by:
- Arrhythmia;
- Weakening of skeletal muscles up to paralysis;
- Apathetic mood;
- Blood pressure instability (jumps from low to high).
Thus, the exchange of “K” is extremely important for maintaining the normal functioning of both each individual cell and the entire organism as a whole. Therefore, if any symptoms appear that may correspond to hypo- or hyperkalemia, it is imperative to take biochemistry tests.
How to lower potassium levels
If the amount of microelement is not controlled, the risk of a heart attack will increase. The first step in dealing with hyperkalemia should be to see a doctor. The specialist must check all medications and dietary supplements that the patient takes and find analogues to drugs that provoke an increase in potassium levels.
If total potassium is slightly increased, the problem can be dealt with without the help of a doctor. It is enough to refuse products with a high content of microelements in their composition. In particular, it is better to replace cow's milk with a natural oat or rice drink. Tomatoes and oranges are other common foods that increase potassium levels in the blood. As an alternative, nutritionists suggest eating grapes, apples or cranberries. You can also use less table salt.
You can get tested quickly and painlessly in our clinic. The technician will take a small amount of blood from a vein. The whole procedure will not take even ten minutes.
Important! During blood sampling for total potassium, it is strictly forbidden to clench your fist. This can lead to pseudohyperkalemia: the release of electrolyte outside the cells, the formation of a blood clot in a test tube. As a result, the analysis results will artificially increase by 1.5-2 mmol/l. At a rate of 3.5 – 5.0 mmol/l, such a “deviation” can be critical.
If your total potassium test results are outside the normal range, it does not necessarily indicate a medical condition that requires immediate medical attention. Some dietary supplements, multivitamins, and even herbs can increase potassium levels in the blood. The clinic specialists will definitely issue a sheet with the results of the analysis. But the indicators must be deciphered by a specialized doctor.
Basic electrolytes
Potassium
- an important component of most cells. Together with other electrolytes, potassium ions are responsible for the functioning of muscles and nerves, normal acid-base balance, and water metabolism. The blood contains only a small amount of the macronutrient; even minor fluctuations in its level lead to serious consequences. Significant deviations from the norm can lead to life-threatening conditions (shock, cardiac dysfunction, respiratory failure, etc.). Normal potassium concentration is 3.5-5.1 mmol/liter.
Magnesium
participates in energy production, enzyme synthesis, muscle contraction and other vital processes. It is absorbed from food into the gastrointestinal tract and excreted by the kidneys. A deficiency of this electrolyte can be caused by poor nutrition, problems with its absorption, or excessive loss in urine. Elevated levels may indicate kidney pathologies. Significant electrolyte deficiency leads to symptoms such as weakness, confusion, loss of appetite, and muscle spasms. Its excess manifests itself in a similar way. Heart rhythm disturbances and nausea may also be added to the symptoms.
Sodium
present in all tissues and fluids of the body. It is necessary for muscle contraction and maintaining water-salt balance. The macroelement is absorbed in the intestines from ordinary table salt. Deviations of its normal level are associated with a violation of one of the mechanisms of its maintenance. For example, normal sodium concentrations are disrupted if antidiuretic hormone, which prevents fluid loss through urine, is produced in abnormal amounts. A violation of the amount of this electrolyte leads to the appearance of edema or dehydration, as the amount of fluid in the tissues changes. Sodium testing is used in the diagnosis of many diseases (for example, pathologies of the kidneys, lungs, brain).
Chlorine
It is part of many biologically active substances and performs a number of physiological functions. Its level is normally relatively stable (a slight decrease in levels is observed after eating). A test for the amount of chlorine is often prescribed in conjunction with other studies to identify various pathologies. Its results are also used to determine the cause of weakness, prolonged vomiting, diarrhea, and breathing problems.
Detailed description of the study
Potassium is one of the main cations (positively charged ions) in the human body. The main part of it is located inside cells and only a small part is in the extracellular space, including blood plasma. Potassium supports the electrical activity of cells, participates in the processes of conducting nerve impulses, muscle contraction, regulating water balance and acid-base balance in the blood.
The concentration of potassium in plasma depends on its intake, redistribution, excretion from the body and is regulated by various substances. In this way, a balance is maintained between intra- and extracellular potassium protein in the cell membrane (sheath) - the potassium-sodium pump. Insulin increases the influx of ions into cells, while alpha adrenergic agonists increase their exit from it. Blood pH also affects the level of this cation. With an increase in acidity (acidosis), potassium is released into the plasma, and with a decrease in acidity (alkalosis), there is an influx into the cell.
The macronutrient enters the body with food: meat, fruits and vegetables. A lot of it is found in apricots, prunes, potatoes, and legumes. The daily norm for humans is 4-5 g. Potassium is absorbed mainly in the small intestine and enters the cells. It is filtered in the kidneys and reabsorbed in large quantities - it enters the blood again. The hormone aldosterone, which is produced by the adrenal glands, increases the excretion of macronutrients by the kidneys. Potassium is also excreted through the intestines and sweat glands.
The concentration of potassium in plasma is quite low and even small fluctuations can lead to disturbances in the body. A test for potassium in the blood plasma may show a decrease in its level (hypokalemia). It occurs due to insufficient intake of the macroelement from food, increased excretion in the urine (some kidney diseases), loss of the ion from intestinal contents (vomiting, diarrhea), and increased production of aldosterone. The main symptoms of hypokalemia are associated with impaired neuromuscular conduction. A person experiences fatigue, muscle weakness, decreased muscle tone, and heart rhythm disturbances.
Hyperkalemia occurs due to excess potassium intake into the body, for example, during intensive infusion of potassium-containing drugs. Also, an increase in the level of the cation in plasma can be observed with insufficient excretion by the kidneys and increased release from cells (destruction of red blood cells, disseminated internal coagulation syndrome and other reasons). Symptoms of hyperkalemia: weakness in the legs, muscle paresis, abdominal pain, numbness, heart rhythm disturbances with a decrease in contraction frequency, and in severe cases, cardiac arrest.
A biochemical blood test for potassium allows you to diagnose its excess or deficiency and take timely measures to correct disorders.