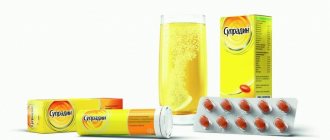
In the autumn-winter period, the level of seasonal incidence of acute respiratory diseases increases steadily. To cope with the unpleasant manifestations of the disease, patients try to use a drug such as “antigrippin”. The drug is available for both adults and children. The medication has an anti-inflammatory effect, very quickly reduces body temperature and eliminates allergic symptoms.
Composition and release form
| Powder for preparing a solution for oral administration, honey-lemon or chamomile | 1 pack |
| paracetamol | 500 mg |
| ascorbic acid | 200 mg |
| chlorphenamine maleate | 10 mg |
| Excipients | |
| honey-lemon powder: sodium bicarbonate; lemon acid; sorbitol; povidone; sucrose; sodium cyclamate; aspartame; acesulfame potassium; lime flavor (aromatic fruit additive “Lime”); caramel flavoring; honey flavoring; docusate sodium; | |
| chamomile powder: sodium bicarbonate; lemon acid; sorbitol; povidone; sucrose; sodium cyclamate; aspartame; acesulfame potassium; chamomile extract; docusate sodium |
in bags made of combined material (paper/Al/PE) 5 g each; in a cardboard pack there are 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 15, 16, 20 or 30 sachets.
| Effervescent tablets with raspberry or grapefruit flavor | 1 table |
| paracetamol | 500 mg |
| ascorbic acid | 200 mg |
| chlorphenamine maleate | 10 mg |
| Excipients | |
| effervescent tablets with raspberry flavor: sodium bicarbonate; lemon acid; sorbitol; povidone; sodium saccharinate; aspartame; sodium carbonate; macrogol; sodium lauryl sulfate; sodium riboflavin 5-phosphate; raspberry flavor (aromatic fruit additive “Raspberry”); taste corrector, red beet juice powder; | |
| effervescent tablets with grapefruit flavor: sodium bicarbonate; lemon acid; sorbitol; povidone; aspartame; sodium carbonate; macrogol; sodium lauryl sulfate; sodium riboflavin 5-phosphate; lemon flavor (aromatic fruit additive “Lemon”); grapefruit flavoring (aromatic fruit additive “Grapefruit”); taste corrector |
in plastic cases 10 pcs.; in a cardboard pack or envelope pack with a device for hanging 1 pencil case; blister 10 pcs. or in a strip 2, 4 or 6 pieces; in a cardboard pack there are 1, 2, 3, 4, 5 blisters or 5, 10, 15, 20 strips.
| Effervescent tablets | 1 table |
| paracetamol | 500 mg |
| ascorbic acid | 200 mg |
| chlorphenamine maleate | 10 mg |
| excipients: sodium bicarbonate; lemon acid; sorbitol; povidone; sodium saccharinate; sodium carbonate; macrogol; sodium lauryl sulfate; lime flavor (aromatic fruit additive “Lime”) |
in plastic cases 10 pcs.; in a cardboard pack or envelope pack with a device for hanging 1 pencil case; blister 10 pcs. or in a strip 2, 4 or 6 pieces; in a cardboard pack there are 1, 2, 3, 4, 5 blisters or 5, 10, 15, 20 strips.
| Effervescent tablets for children | 1 table |
| paracetamol | 250 mg |
| ascorbic acid | 50 mg |
| chlorphenamine maleate | 3 mg |
| excipients: sodium bicarbonate; lemon acid; sorbitol; povidone; sodium saccharinate; sodium carbonate; macrogol; silicon dioxide; fruit flavoring (aromatic fruit additive “Red Fruits”) |
in plastic cases 10 pcs.; in a cardboard pack or envelope pack with a device for hanging 1 pencil case; blister 10 pcs. or in a strip 2, 4 or 6 pieces; in a cardboard pack there are 1, 2, 3, 4, 5 blisters or 5, 10, 15, 20 strips.
Compound
| Set of capsules | 1 kit |
| Capsule A | 1 caps. |
| active substances: | |
| ascorbic acid | 300 mg |
| acetylsalicylic acid | 250 mg |
| rutoside trihydrate (in terms of rutoside) | 20 mg |
| excipients: calcium stearate - 1 mg; potato starch - 9 mg | |
| hard gelatin capsule: gelatin - 90.723 mg; dye azorubine (E122) - 0.003 mg; brilliant black dye (E151) - 0.184 mg; patented blue dye (E131) or brilliant blue dye (E133) - 0.315 mg; quinoline yellow dye (E104) - 2.207 mg; titanium dioxide (E171) - 2.56 mg | |
| Capsule B | 1 caps. |
| active substances: | |
| metamizole sodium monohydrate | 250 mg |
| calcium gluconate monohydrate | 100 mg |
| diphenhydramine hydrochloride | 20 mg |
| excipients: calcium stearate - 3.8 mg; potato starch - 6.2 mg | |
| hard gelatin capsule: gelatin - 91.944 mg; titanium dioxide (E171) – 4.055 mg |
Description of the dosage form
Powder for preparing a solution for oral administration, honey-lemon: powder of varying degrees of granulation, consisting of particles from white to grayish-beige in color, with a specific odor. Dark brown inclusions are allowed.
Chamomile powder for the preparation of a solution for oral administration: powder of varying degrees of granulation, consisting of particles from white to beige and light brown, with a specific odor. Brown inclusions are allowed.
Effervescent tablets with raspberry flavor: round, flat, with a beveled edge and a separating line on one side, pink, pinkish-lilac or lilac in color, with lighter and darker inclusions, with a specific fruity odor.
Effervescent tablets with grapefruit flavor: round, flat, with a beveled edge and a scoring line on one side, white, almost white or creamy-white in color, with barely noticeable marbling, with a specific citrus odor.
Effervescent tablets: round, flat, with a beveled edge and a score line on one side, white or almost white in color, with barely noticeable marbling, with a fruity odor.
Effervescent tablets for children: round, flat, with a beveled edge and a separating line on one side, pink in color with lighter and darker inclusions, randomly located, with a fruity smell.
Pharmacodynamics
Combined drug.
Paracetamol has an analgesic and antipyretic effect, eliminates headaches and other types of pain, and reduces fever.
Ascorbic acid (vitamin C) - participates in the regulation of redox processes, carbohydrate metabolism, and increases the body's resistance.
Chlorphenamine - a blocker of H1-histamine receptors - has an antiallergic effect, facilitates breathing through the nose, reduces nasal congestion, sneezing, lacrimation, itching and redness of the eyes.
What is it made of?
"Antigrippin" includes several main active ingredients. The first active substance is paracetamol. It helps lower body temperature and has an analgesic effect. The second component of the drug is chlorphenamine maleate. It is aimed at combating allergic reactions of the patient's body. In addition, this substance alleviates the clinical manifestations and course of rhinitis. The third element in the composition is ascorbic acid. It plays an important role in the occurrence of redox reactions in various processes. In addition, it stimulates the immune system to increase natural resistance to various infectious diseases.
In general, the medication is an effervescent tablet, round in shape, usually available in three different flavors (raspberry, lemon, grapefruit). The color of the pill changes depending on the taste. In addition to the main components, the drug includes a significant list of additional substances.
Contraindications
Common to four dosage forms:
hypersensitivity to paracetamol, ascorbic acid, chlorphenamine or any other component of the drug;
erosive and ulcerative lesions of the gastrointestinal tract (in the acute phase);
severe renal and/or liver failure;
angle-closure glaucoma.
For powder for preparing a solution for oral administration of honey-lemon or chamomile, effervescent tablets with raspberry or grapefruit flavor, effervescent tablets (optional):
alcoholism;
phenylketonuria;
prostatic hyperplasia;
children under 15 years of age;
pregnancy;
lactation period.
For effervescent tablets for children (optional) - children under 3 years of age.
Carefully
For four dosage forms:
renal and/or liver failure;
deficiency of glucose-6-phosphate dehydrogenase;
congenital hyperbilirubinemia (Gilbert, Dubin-Johnson and Rotor syndromes);
viral hepatitis.
For powder for preparing a solution for oral administration of honey-lemon or chamomile, effervescent tablets with raspberry or grapefruit flavor, effervescent tablets (optional):
alcoholic hepatitis;
elderly age;
hyperoxalaturia (effervescent tablets with raspberry or grapefruit flavor);
progressive malignant diseases (effervescent tablets with raspberry or grapefruit flavor).
When not to
Antigrippin has a fairly large list of contraindications. The drug is prohibited for use in persons suffering from erosive and ulcerative diseases of the gastrointestinal tract in acute cases and during periods of exacerbations. Kidney and liver failure, viral and alcoholic hepatitis are also a stop signal for taking this drug. The medication is strictly prohibited in case of alcohol abuse, the presence of closed-angle glaucoma, phenylketonuria, as well as pregnant women and during the lactation period. The presence of prostatic hyperplasia will not be an exception. Caution should be exercised when prescribing these tablets to older people.
Side effects
The drug is well tolerated in recommended doses.
In isolated cases there are
From the side of the central nervous system: headache, feeling of fatigue.
From the gastrointestinal tract: nausea, pain in the epigastric region.
From the endocrine system: hypoglycemia (up to the development of coma).
From the hematopoietic organs: anemia, hemolytic anemia (especially for patients with glucose-6-phosphate dehydrogenase deficiency); extremely rarely - thrombocytopenia.
Allergic reactions: skin rash, itching, urticaria, Quincke's edema.
Other: hypervitaminosis, metabolic disorders, feeling of heat, dry mouth, accommodation paresis, urinary retention, drowsiness.
For powder for the preparation of a solution for oral administration of honey-lemon, effervescent tablets with raspberry or grapefruit flavor (optional): anaphylactoid reactions (including anaphylactic shock), exudative erythema multiforme (including Stevens-Johnson syndrome), toxic epidermal necrolysis (Lyell's syndrome).
All side effects of the drug should be reported to your doctor.
Antigrippin No. 10 tablet.
APPROVED by the Order of the Chairman of the Committee for Control of Medical and Pharmaceutical Activities of the Ministry of Health of the Republic of Kazakhstan dated “____”_________20__. No. ______________ INSTRUCTIONS for the medical use of the drug Antigrippin Trade name Antigrippin International nonproprietary name No Dosage form Tablets Composition One tablet contains active substances: ascorbic acid - 0.30 g, calcium gluconate - 0.10 g, diphenhydramine - 0.02 g, rutin - 0.02 g excipients: starch, talc, calcium stearate. Description Tablets of greenish-yellow color with slight inclusions, with a flat surface, with a chamfer and a score. Pharmacotherapeutic group To eliminate the symptoms of colds and coughs. Other combinations of drugs to relieve cold symptoms. ATX code R05X Pharmacological properties The pharmacological activity of the drug is determined by the properties of the active substances included in its composition. Pharmacokinetics Ascorbic acid is well absorbed after oral administration. About 25% binds to plasma proteins, is deposited in plasma and cells, the highest concentration is achieved in glandular tissues (mainly in the adrenal cortex and pituitary gland). Metabolized in the liver, excreted in the urine in the form of oxalate and unchanged. After oral administration, about 30% of ionized calcium is absorbed into the gastrointestinal tract. After oral administration, the maximum concentration in the blood plasma is reached after 1.2–1.3 hours. It is excreted from the body mainly in feces (80%) and urine (20%). Diphenhydramine is quickly absorbed from the gastrointestinal tract. Bioavailability is 50%. Plasma protein binding – 98-99%. Penetrates through the BBB. Metabolized mainly in the liver, partially in the lungs and kidneys. Within 24 hours, it is completely excreted by the kidneys in the form of metabolites. It is also excreted in milk and may cause sedation in infants. The maximum concentration of rutin after oral administration is reached after 1-9 hours. It is excreted mainly with bile and to a lesser extent by the kidneys. Pharmacodynamics Ascorbic acid replenishes vitamin C deficiency in the body, has pronounced antioxidant properties, regulates redox processes, and increases the body's resistance to infections. Calcium gluconate has an antiallergic, hemostatic effect, reduces the fragility and permeability of blood vessels, symptoms of calcium deficiency in the body, improves muscle contraction in muscular dystrophy, myasthenia gravis. Diphenhydramine has antiallergic activity, has a local anesthetic, antispasmodic and moderate ganglion-blocking effect. When taken orally, it causes a sedative and hypnotic effect and has a moderate antiemetic effect. The angioprotector rutin belongs to the vitamin P group; in combination with ascorbic acid, it reduces the permeability and fragility of capillaries, participates in redox processes, and has antioxidant properties. Indications for use Symptomatic treatment of influenza and other acute respiratory viral infections. Method of administration and dosage: Adults are prescribed 1 tablet orally 3 times a day; children over 7 years old: ½ tablet 3 times a day after meals for 3-5 days. The maximum single dose for adults is 2 tablets, the daily dose is 6 tablets; for children over 7 years old – 1 tablet and 3 tablets, respectively. It is not recommended to use the drug for more than 5 days without consulting a doctor. If symptoms persist, you should consult a doctor. Do not exceed recommended doses. Side effects - dyspeptic symptoms, epigastric pain, nausea, vomiting, dry mouth - headache, feeling tired, drowsiness, increased excitability of the central nervous system, sleep disturbance, decreased speed of psychomotor reactions; in children, diphenhydramine can cause the paradoxical development of insomnia, irritability and euphoria - with long-term use in high doses, inhibition of the function of the insular apparatus of the pancreas (hyperglycemia, glycosuria), hyperoxaluria and the formation of urinary stones from calcium oxalate is possible - decreased capillary permeability and deterioration of tissue trophism - thrombocytosis, thrombocytopenia, leukopenia, neutropenia, agranulocytosis, anemia , hemolytic anemia (especially for patients with glucose-6-phosphate dehydrogenase deficiency), hyperprothrombinemia, erythropenia, neutrophilic leukocytosis, hypokalemia - allergic reactions: urticaria, itching, skin hyperemia - difficulty urinating (especially in men with an enlarged prostate gland) - increased viscosity secretions of the respiratory tract All side effects, including those not listed above, should be reported to your doctor and stop taking the drug. Contraindications - hypersensitivity to the components of the drug - epilepsy - children under 7 years of age - pregnancy and lactation Drug interactions Interactions with drugs occur more often with long-term use of large doses of ascorbic acid. Ascorbic acid increases blood concentrations of salicylates (increases the risk of crystalluria), ethinyl estradiol, benzylpenicillin and tetracyclines. With estrogens - the level of the hormone in the blood serum increases. With oral contraceptives containing estrogens, the contraceptive effect is reduced. Reduces the anticoagulant effect of coumarin derivatives. Improves the absorption of iron preparations in the intestines. Increases the total clearance of ethyl alcohol. Quinoline drugs, calcium chloride, salicylates, and corticosteroids deplete ascorbic acid reserves when used for a long time. Acetylsalicylic acid, oral contraceptives, fresh juices and alkaline drinks reduce the absorption and absorption of ascorbic acid. With the simultaneous use of ascorbic acid with isoprenaline, the chronotropic effect of the latter decreases. In high doses, it increases the excretion of mexiletine by the kidneys. Barbiturates and pyrimidine increase the excretion of ascorbic acid in the urine. Ascorbic acid reduces the therapeutic effect of antipsychotic drugs (neuroleptics) - phenothiazine derivatives, tubular reabsorption of amphetamine and tricyclic antidepressants. Calcium gluconate. Due to the possibility of the formation of non-absorbable complexes, calcium may reduce the absorption of estramustine, etidronate and possibly other bisphosphonates, phenytoin, quinolones, oral tetracycline antibiotics and drugs should be given at least 3 hours. , spinach, rhubarb, bran and grains. When prescribing high doses of calcium to patients receiving digitalis preparations, it may increase the risk of arrhythmias. Thiazide diuretics reduce urinary calcium excretion. Therefore, the risk of hypercalcemia should be kept in mind when using them simultaneously. Calcium may reduce the absorption of tetracycline antibiotics and fluoride preparations when taken simultaneously. Concomitant use of vitamin D increases calcium absorption. With simultaneous use, diphenhydramine enhances the effect of ethanol and drugs that depress the central nervous system, barbiturates, hypnotics, opiate analgesics. Therefore, when using these drugs together, you should consult your doctor to avoid potentiated effects. MAO inhibitors enhance the anticholinergic activity of diphenhydramine. Antagonistic interactions are observed when co-administered with psychostimulants. Reduces the effectiveness of apomorphine as an emetic in the treatment of poisoning. Enhances the anticholinergic effects of drugs with anticholinergic activity. The pharmacological effect of rutin is enhanced by ascorbic acid. Special instructions During the treatment period, you should avoid drinking alcohol. Do not combine with taking sleeping pills. Given the stimulating effect of ascorbic acid on the synthesis of corticosteroid hormones, it is necessary to monitor kidney function and blood pressure. Ascorbic acid in patients with rapidly proliferating and intensively metastasizing tumors can aggravate the course of the disease. Ascorbic acid can distort the results of various laboratory tests (determination of glucose, bilirubin and liver transaminase activity, LDH in blood plasma). Ascorbic acid is prescribed with caution to patients with renal failure, or for diseases associated with elevated levels of vitamin D, diseases such as sarcoidosis. All side (unusual) effects, including those not listed above, should be reported to your doctor. If you do not feel better, you should stop taking the drug and consult a doctor. If the patient is undergoing surgery, he must notify the doctor in advance about taking the drug. Pregnancy and lactation. The use of the drug is not recommended. Peculiarities of the drug's influence on the ability to drive a vehicle or operate potentially dangerous mechanisms. Use with caution in patients engaged in potentially hazardous activities that require increased attention and rapid psychomotor reactions. Overdose Symptoms: depression of the central nervous system, development of agitation (especially in children) or depression, dilated pupils, dry mouth, convulsions. Signs of hypercalcemia: anorexia, nausea, vomiting, impaired consciousness, nephrocalcinosis, calciuria and, in severe cases, arrhythmia and coma. Treatment: gastric lavage with water with activated carbon and symptomatic therapy. Forced diuresis, hemodialysis. Release form and packaging Tablets of 10 pieces in a contour-free packaging made of packaging paper with a polymer coating on both sides. 250 contour packages together with instructions for medical use in the state and Russian languages are placed in a cardboard box (multiple packaging). Storage conditions Store in a dry place, protected from light, at a temperature not exceeding 25 °C. Keep out of the reach of children! Shelf life 2 years. Do not use the drug after the expiration date. Conditions for dispensing from pharmacies By prescription, Republic of Kazakhstan, Almaty region, village. Boraldai, 71 crossing point. Owner of the registration certificate Eikos-Pharm LLP, Republic of Kazakhstan, Almaty region, village. Boraldai, 71 crossing point. Address of the organization that receives complaints from consumers regarding the quality of products. Almaty, st. Nusupbekova, 32 tel: 397 64 29, fax: 250 71 78 e-mail
Interaction
For four dosage forms
Ethanol enhances the sedative effect of antihistamines.
Antidepressants, antiparkinsonian drugs, antipsychotic drugs (phenothiazine derivatives): increase the risk of side effects (urinary retention, dry mouth, constipation).
GCS: increase the risk of developing glaucoma.
With simultaneous use, the positive chronotropic effect of isoprenaline decreases.
Reduces the therapeutic effect of antipsychotic drugs (neuroleptics) - phenothiazine derivatives, tubular reabsorption of amphetamine and tricyclic antidepressants.
Inducers of microsomal oxidation in the liver (phenytoin, ethanol, barbiturates, rifampicin, phenylbutazone, tricyclic antidepressants): increase the production of hydroxylated active metabolites, which makes it possible to develop severe intoxications with small overdoses. Ethanol contributes to the development of acute pancreatitis.
Inhibitors of microsomal oxidation (including cimetidine): reduce the risk of hepatotoxicity.
Simultaneous use of the drug Antigrippin and diflunisal: the concentration in the blood plasma of paracetamol increases by 50%, resulting in increased hepatotoxicity.
Simultaneous use of barbiturates reduces the effectiveness of paracetamol and increases the excretion of ascorbic acid in the urine.
Paracetamol: reduces the effectiveness of uricosuric drugs.
For powder for preparing a solution for oral administration of honey-lemon and chamomile, effervescent tablets with raspberry or grapefruit flavor (optional)
Ascorbic acid: increases the concentration of benzylpenicillin and tetracyclines in the blood; improves the absorption of iron preparations in the intestine (converts ferric iron to divalent iron); may increase iron excretion when used concomitantly with deferoxamine; increases the risk of developing crystalluria during treatment with salicylates and short-acting sulfonamides, slows down the excretion of acids by the kidneys, increases the excretion of drugs that have an alkaline reaction (including alkaloids), reduces the concentration of oral contraceptives in the blood; increases the overall clearance of ethanol; can both increase and decrease the effect of anticoagulant drugs.
Chlorphenamine maleate: enhances the effect of hypnotics.
Antigrippin No. 10 adult effervescent tablets
Compound:
Paracetamol - 500 mg, Ascorbic acid - 200 mg, Chlorphenamine maleate - 10 mg. Other ingredients: sodium bicarbonate, anhydrous citric acid, sorbitol (E 420), povidone, sodium saccharin, anhydrous sodium carbonate, macrogol, sodium lauryl sulfate, aromatic fruit additive "Lime".
Pharmacological properties:
Combined drug. Paracetamol has an analgesic and antipyretic effect, eliminates headaches and other types of pain, and reduces elevated body temperature. Ascorbic acid (vitamin C) is involved in the regulation of redox processes, carbohydrate metabolism, and increases the body's resistance. Chlorphenamine - a blocker of H1-histamine receptors - has an antiallergic effect, facilitates breathing through the nose, reduces the feeling of nasal congestion, sneezing, tearing, itching and inflammation of the eyes. The drug is well absorbed from the gastrointestinal tract, and the Cmax of paracetamol in the blood plasma is determined within 30–60 minutes. Delayed excretion of paracetamol and its metabolites is observed in cases of impaired renal function and chronic liver diseases.
Indications:
Short-term symptomatic treatment of flu and colds, including headache, muscle and joint pain, sore throat, fever, nasal congestion. Release form: 10 effervescent tablets in a plastic tube.
Mode of application:
Inside. Adults and children over 15 years old, 1 tablet 2-3 times a day. The AntiGrippin tablet should be completely dissolved in a glass (200 ml) of warm water (50-60°C) and the resulting solution should be drunk immediately. It is better to take the drug between meals. The maximum daily dose is 3 tablets. The interval between doses of the drug should be at least 4 hours. In patients with impaired liver or kidney function and in elderly patients, the interval between doses of the drug should be at least 8 hours. The duration of taking AntiGrippin without consulting a doctor is no more than 5 days when prescribed as an analgesic and 3 days as an antipyretic.
Contraindications:
Hypersensitivity to the components of the drug or other antihistamines, severe dysfunction of the liver and/or kidneys, congenital hyperbilirubinemia, glucose-6-phosphate dehydrogenase deficiency, alcoholism, blood diseases, severe anemia, leukopenia, severe hypertension, unstable angina, severe cardiac conduction disorders, acute period of myocardial infarction, severe atherosclerosis, uncompensated heart failure, hyperthyroidism, acute urinary retention with prostatic hypertrophy, obstruction of the bladder neck, pyloroduodenal obstruction, gastric and duodenal ulcers in the acute stage, angle-closure glaucoma, thrombosis, thrombophlebitis, severe forms of diabetes mellitus , epilepsy, old age; fructose intolerance; phenylketonuria. Do not use together with MAO inhibitors and within 2 weeks after discontinuation of MAO inhibitors. Contraindicated in patients taking tricyclic antidepressants or β-adrenergic receptor blockers for urolithiasis, provided that ascorbic acid enters the body in a dose of more than 1 g/day.
Side effects
In recommended doses, the drug is well tolerated. Possible in isolated cases: from the central nervous system: headache, feeling of fatigue; from the gastrointestinal tract: nausea, pain in the epigastric region; from the endocrine system: hypoglycemia (up to the development of coma); from the hematopoietic organs: anemia, hemolytic anemia (especially for patients with glucose-6-phosphate dehydrogenase deficiency); extremely rarely - thrombocytopenia; allergic reactions: skin rash, itching, urticaria, Quincke's edema, anaphylactoid reactions (including anaphylactic shock), exudative erythema multiforme (including Stevens-Johnson syndrome), toxic epidermal necrolysis (Lyell's syndrome); other: hypervitaminosis C, metabolic disorders, feeling of heat, dry mouth, accommodation paresis, urinary retention, drowsiness. All side effects of the drug should be reported to your doctor.
Interaction
It can either increase or decrease the effect of anticoagulant drugs. Reduces the therapeutic effect of antipsychotic drugs (neuroleptics) - phenothiazine derivatives, tubular reabsorption of amphetamine and tricyclic antidepressants. Concomitant use of barbiturates increases the excretion of ascorbic acid in the urine. Chlorphenamine maleate Chlorphenamine maleate enhances the effect of hypnotics. Antidepressants, antiparkinsonian drugs, antipsychotic drugs (phenothiazine derivatives) - increase the risk of side effects (urinary retention, dry mouth, constipation). Glucocorticosteroids - increase the risk of developing glaucoma. Ethanol enhances the sedative effect of chlorphenamine maleate. Paracetamol When paracetamol interacts with inducers of microsomal oxidation in the liver (phenytoin, ethanol, barbiturates, rifampicin, phenylbutazone, tricyclic antidepressants), the production of hydroxylated active metabolites increases, which makes it possible to develop severe intoxications with small overdoses. While taking paracetamol, ethanol contributes to the development of acute pancreatitis. Inhibitors of microsomal oxidation (including cimetidine) reduce the risk of hepatotoxicity. The simultaneous use of diflunisal and paracetamol increases the plasma concentration of the latter by 50%, increasing hepatotoxicity. Concomitant use of barbiturates reduces the effectiveness of paracetamol. Paracetamol reduces the effectiveness of uricosuric drugs. Ascorbic acid increases the concentration of benzylpenicillin and tetracyclines in the blood; improves the absorption of iron preparations in the intestine (converts ferric iron to divalent iron); may increase iron excretion when used concomitantly with deferoxamine; increases the risk of developing crystalluria during treatment with salicylates and short-acting sulfonamides, slows down the excretion of acids by the kidneys, increases the excretion of drugs that have an alkaline reaction (including alkaloids), and reduces the concentration in the blood of oral contraceptives. increases the overall clearance of ethanol; when used simultaneously, it reduces the chronotropic effect of isoprenaline.
Special instructions
You should consult your doctor if you are taking metoclopramide, domperidone or cholestyramine. Under the influence of paracetamol and ascorbic acid, laboratory test parameters (quantitative determination of glucose and uric acid in blood plasma, bilirubin, activity of “liver” transaminases, LDH) may be distorted. With long-term use in doses significantly higher than recommended, the likelihood of impaired liver and kidney function increases; monitoring of the peripheral blood picture is necessary. To avoid toxic liver damage, paracetamol should not be combined with alcoholic beverages, or taken by persons prone to chronic alcohol consumption. The risk of developing liver damage increases in patients with alcoholic hepatosis. Prescribing ascorbic acid to patients with rapidly proliferating and intensively metastasizing tumors can aggravate the process. In patients with high iron levels in the body, ascorbic acid should be used in minimal doses.
Storage conditions
At a temperature of 10-30°C. Keep out of the reach of children!
Best before date
3 years. Do not use AntiGrippin after the expiration date.
Directions for use and doses
Inside.
Powder for preparing a solution for oral administration, honey-lemon or chamomile, effervescent tablets with raspberry or grapefruit flavor, effervescent tablets: adults and children over 15 years old - 1 pack. or 1 table. 2–3 times a day. The maximum daily dose is 3 packs. or 3 tables
Effervescent tablets for children: from 3 to 5 years - 1/2 tablet. 2 times a day, from 5 to 10 years - 1 tablet. 2 times a day, from 10 to 15 years - 1 tablet. 2–3 times a day.
The contents of the sachet or tablet should be completely dissolved in a glass (200 ml) of warm water (50–60 °C) and the resulting solution should be drunk immediately. It is better to take the drug between meals. The interval between doses of the drug should be at least 4 hours.
In patients with impaired liver or kidney function and in elderly patients, the interval between doses of the drug should be at least 8 hours.
The duration of use without consulting a doctor is no more than 5 days when prescribed as an analgesic and 3 days when prescribed as an antipyretic.
Overdose
Symptoms: caused by the substances included in the composition. The clinical picture of an acute overdose of paracetamol develops within 6–14 hours after its administration. Symptoms of chronic overdose appear 2–4 days after increasing the dose of the drug.
Symptoms of acute paracetamol overdose: diarrhea, loss of appetite, nausea and vomiting, abdominal discomfort and/or abdominal pain, increased sweating.
Symptoms of an overdose of chlorphenamine: dizziness, agitation, sleep disturbance, depression, convulsions.
Treatment: symptomatic.
special instructions
For four dosage forms
If you are taking metoclopramide, domperidone or cholestyramine, you should consult your doctor.
With long-term use in doses significantly higher than recommended, the likelihood of liver and kidney dysfunction increases; monitoring of the peripheral blood picture is necessary.
Paracetamol and ascorbic acid can distort laboratory tests (quantitative determination of glucose and uric acid in blood plasma, bilirubin, liver transaminase activity, LDH).
Prescribing ascorbic acid to patients with rapidly proliferating and intensively metastasizing tumors can aggravate the process. In patients with high iron levels in the body, ascorbic acid should be used in minimal doses.
For powder for preparing a solution for oral administration of honey-lemon or chamomile, effervescent tablets with raspberry or grapefruit flavor, effervescent tablets (optional)
To avoid toxic liver damage, paracetamol should not be combined with alcoholic beverages, or taken by persons prone to chronic alcohol consumption. The risk of developing liver damage increases in patients with alcoholic hepatosis.
For powder for preparing a solution for oral administration of honey-lemon or chamomile (optional)
One sachet of honey-lemon powder contains 1.793 g of sugar, which corresponds to 0.15 XE.
One sachet of chamomile powder contains 2.058 g of sugar, which corresponds to 0.17 XE.
Antigrippin effervescent tablets with raspberry flavor No. 10 in a plastic case
Name
Antigrippin tablet. thorn. with taste. raspberries in plastic foam No. 10 in an envelope pack. No. 1
Description
The tablets are round, flat, with a bevel and a separating line on one side, pink, pinkish-lilac or lilac in color, with lighter and darker inclusions.
Main active ingredient
Paracetamol+chlorphenamine+ascorbic acid
Release form
Effervescent tablets
Dosage
paracetamol - 325 mg chlorphenamine maleate - 10 mg ascorbic acid - 200 mg
Pharmacological properties
A combination drug consisting of three active substances. Paracetamol has an analgesic and antipyretic effect; eliminates headaches and other types of pain, reduces fever. Chlorphenamine maleate is an antihistamine, a blocker of H1-histamine receptors, a propylamine derivative, and has an anticholinergic effect. Antihistamines exhibiting H1-histaminolytic properties have the ability of reversible competitive antagonism with histamine in the tissues of the skin, lungs, intestines and blood vessels. The sedative effect of chlorphenamine maleate is due to its penetration through the blood-brain barrier. The adrenolytic properties of chlorphenamine maleate may increase the risk of orthostatic hypotension. It has an antiallergic effect, helps restore nasal breathing, reducing swelling of the nasal mucosa and mucus production, lacrimation and runny nose. Ascorbic acid (vitamin C) is involved in the regulation of redox processes, carbohydrate metabolism, and increases the body's resistance.
Indications for use
In adults and children over 15 years of age, to relieve symptoms associated with colds and flu, such as clear nasal discharge, watery eyes, sneezing, headache, muscle pain, and/or fever (increased body temperature).
Directions for use and doses
Inside. Adults and children over 15 years old, 1 tablet 1-2 times a day. The tablet should be completely dissolved in a glass (200 ml) of warm water (50-60 °C) and drink the resulting solution immediately. It is better to take the drug between meals. The maximum daily dose is 2 tablets. The interval between doses of the drug should be at least 4 hours. In patients with impaired liver and kidney function, the interval between doses of the drug should be at least 8 hours (see also “Contraindications”, “Precautions”). In elderly patients: the maximum daily dose is 1 tablet (see "Precautions"). If the temperature does not decrease after 3 days of taking Antigrippin and/or the symptoms of the disease do not improve, you should consult a doctor. The maximum duration of use as an anesthetic is 5 days. The drug is not suitable for relieving pain not associated with colds, as it contains components that together eliminate the symptoms of a cold.
Use during pregnancy and lactation
The presence of chlorphenamine maleate, as well as ascorbic acid, determines restrictions on use in this category of patients. There are no sufficient data on the safety of use. Antigrippin is contraindicated during pregnancy and breastfeeding.
Precautionary measures
If there is a high fever, signs of superinfection, or if symptoms do not resolve within five days, treatment should be reconsidered. To avoid the risk of overdose, you must make sure that there is no paracetamol, chlorphenamine maleate and ascorbic acid in other medicines (see also section “Interaction with other medicines”). With prolonged use of high doses or improper use of analgesics, headaches may occur that should not be relieved by taking even higher doses of painkillers. Frequent use of analgesics, especially when several of them are combined, leads to the risk of kidney damage and the occurrence of renal failure. If suddenly discontinued after long-term use of high doses of analgesics, headaches, fatigue, muscle pain, nervousness and autonomic symptoms may occur, which disappear within a few days after stopping the drug. Until they disappear, you should avoid taking painkillers; their subsequent use is recommended after consultation with your doctor. - Do not exceed the recommended doses! Currently, there are recommendations to reduce the daily dose of paracetamol from 4 g/day to 3 g/day. Cases of acute liver failure (in some cases resulting in liver transplantation or death) due to the use of paracetamol have been reported. Most cases of liver damage are associated with doses greater than 4 g per day, and are also common with taking more than one paracetamol-containing product. Excessive consumption of paracetamol may be intentional or unintentional, for example when trying to get maximum effect. Patients should consult a doctor immediately if more than 4 g of paracetamol has been taken at one time, even if they feel well. With long-term use in doses significantly higher than recommended, the likelihood of impaired liver and kidney function increases; monitoring of the peripheral blood picture is necessary. To avoid toxic liver damage, paracetamol should not be combined with alcoholic beverages, or taken by persons prone to chronic alcohol consumption. The risk of developing liver damage increases in patients with alcoholic hepatosis. Patients who consume three or more servings of alcohol per day (1 serving: 10-12 g of alcohol = 1 glass of vodka or cognac (25-30 ml) = 1 glass of wine (100-120 ml) = 1 small glass of beer (220- 260 ml)), should be informed of the need to consult with your doctor about when and how to take paracetamol. Chronic alcohol drinkers are at increased risk of liver damage when taking paracetamol, even at recommended doses. Chlorphenamine maleate should be used with caution, especially together with other drugs that have anticholinergic effects, in epilepsy, increased intraocular pressure, severe cardiovascular disease or hypertension, chronic bronchitis, bronchiectasis or bronchial asthma. Neurological anticholinergic effects and paradoxical arousal (eg, hyperactivity, restlessness, nervousness) are more common in children and the elderly. Monitoring of the use of chlorphenamine maleate should be enhanced in elderly patients (high likelihood of orthostatic hypotension, dizziness, sedation, chronic constipation and the risk of paralytic ileus, worsening prostatic hypertrophy), with impaired liver and/or kidney function due to possible accumulation . Prescribing ascorbic acid to patients with rapidly proliferating and intensively metastasizing tumors can aggravate the process. In patients with high iron levels in the body, ascorbic acid should be used in minimal doses. You should not take large doses of ascorbic acid (more than 500 mg) for diabetes mellitus, hyperoxaluria, thalassemia, hemochromatosis, sideroblastic anemia. Due to the content of ascorbic acid, special care should be taken when prescribing to patients with increased blood clotting, polycythemia, leukemia, thrombophlebitis or a tendency to thrombosis, hyperoxaluria, and kidney stones. In conditions that may be accompanied by hyperglycemia, ascorbic acid can be taken only on the recommendation of a doctor and in minimal dosages. Taking large doses of ascorbic acid is associated with the formation of calcium oxalate stones in the kidneys, so ascorbic acid should be used with caution in patients with hyperoxaluria. When taking large doses of ascorbic acid (4 g per day) in patients with glucose-6-phosphate dehydrogenase deficiency, severe hemolysis was observed in some cases. Since the simultaneous use of ascorbic acid with antacids containing aluminum may increase the excretion of aluminum in the urine, their combined use in patients with renal failure is not recommended. Effect on diagnostic tests Abnormally high concentrations of paracetamol may distort the results of blood glucose tests performed by the glucose oxidase-peroxidase method. The use of paracetamol may affect the results of determining blood urea by a method that uses phosphotungstic acid. Ascorbic acid can also distort the parameters of laboratory tests (quantitative determination of glucose and uric acid in the blood plasma, bilirubin, activity of “liver” transaminases - ALT, AST, LDH). This medicine contains sorbitol, which should be taken into account in patients with sugar intolerance. Aspartame is a source of phenylalanine, do not use in patients with phenylketonuria! One dose of Antigrippin contains about 181 mg (8 mmol) Na+, which should be taken into account in patients on a low sodium diet.
Interaction with other drugs
Paracetamol The interaction of paracetamol and inducers of microsomal oxidation in the liver (phenytoin, barbiturates, rifampicin, carbamazepine, ethanol) increases the production of hydroxylated active metabolites, which makes it possible to develop severe intoxications with small overdoses. Concomitant use of barbiturates reduces the effectiveness of paracetamol. The rate of absorption of paracetamol increases when used simultaneously with metoclopramide and domperidone, and decreases when taken together with drugs that slow down gastric emptying (propantheline, antidepressants with anticholinergic properties, narcotic analgesics) and cholestyramine. When taken simultaneously with paracetamol, the half-life of chloramphenicol increases by 5 times. Paracetamol may reduce the effectiveness of lamotrigine. Salicylamide prolongs the half-life of paracetamol and leads to the accumulation of hepatotoxic metabolites. The simultaneous use of zidovudine and paracetamol increases the risk of neutropenia. Probenecid inhibits the conjugation of paracetamol with glucuronic acid and thus leads to a decrease in the clearance of paracetamol. Paracetamol in high doses (4 g/day) for more than 4 days may potentiate the effect of oral anticoagulants and, therefore, increase the risk of bleeding. Constant monitoring of the INR (international normalized ratio) is necessary. It is necessary to consider the use of paracetamol when co-administered with oral anticoagulants and the discontinuation of paracetamol. Chlorphenamine maleate Chlorphenamine maleate can enhance the depressant effect on the central nervous system of many drugs and substances, slowing reaction speed and reducing concentration. These are morphine derivatives (analgesics, antitussives), neuroleptics, tranquilizers, barbiturates, benzodiazepines, sleeping pills, sedative antidepressants (amitriptyline, mianserin, mirtazapine, timipramine), Hi-blockers with sedative action, centrally acting antihypertensives, baclofen, thalidomide. Ethanol enhances the sedative effect of chlorphenamine maleate. Ascorbic acid Increases the concentration of benzylpenicillin and tetracyclines in the blood, improves the absorption of iron in the intestines (converts ferric iron to ferrous), and can increase the excretion of iron when used simultaneously with deferoxamine. Concomitant use of ascorbic acid with antacids containing aluminum may increase urinary excretion of aluminum. When treating with salicylates and short-acting sulfonamides, the joint administration of vitamin C slows down the excretion of acids by the kidneys, increases the excretion of drugs that have an alkaline reaction (including alkaloids), and increases the risk of developing crystalluria. Ascorbic acid, when used simultaneously, reduces the blood concentration of oral contraceptives and indirect anticoagulants, increases the overall clearance of ethanol, reduces the therapeutic effect of isoprenaline, vitamin B12, neuroleptics - phenothiazine derivatives (for example, fluphenazine), reduces the tubular reabsorption of tricyclic antidepressants. Concomitant use of barbiturates increases the excretion of ascorbic acid in the urine. Indomethacin may reduce the effectiveness of ascorbic acid.
Contraindications
Hypersensitivity to paracetamol, ascorbic acid, chlorphenamine or any of the excipients, phenylketonuria, sugar intolerance; deficiency of glucose-6-phosphate dehydrogenase; erosive and ulcerative lesions of the gastrointestinal tract (in the acute phase); severe liver or kidney failure; angle-closure glaucoma; risk of urinary retention associated with prostatic hypertrophy; combined use with monoamine oxidase inhibitors (MAO) and for 2 weeks after their discontinuation; chronic alcohol abuse; children's age (up to 15 years); pregnancy, lactation; Urolithiasis Use with caution in the presence of diseases or conditions such as: epilepsy, bleeding disorders, hyperoxaluria, thalassemia, hemochromatosis, sideroblastic anemia, progressive malignancies, congenital hyperbilirubinemia (Gilbert syndrome, etc.), diabetes mellitus, liver and kidney diseases, renal mild to moderate insufficiency, severe cardiovascular disease or arterial hypertension, chronic bronchitis, bronchiectasis or bronchial asthma, obstruction of the bladder neck, pyloroduodenal obstruction, polycythemia, leukemia, thrombophlebitis, a tendency to thrombosis, as well as in children and elderly patients ( see also section "Precautions").
Compound
For one tablet Active ingredients: paracetamol - 325 mg, chlorphenamine maleate - 10 mg, ascorbic acid - 200 mg
Overdose
Symptoms of an overdose of chlorphenamine maleate: dizziness, agitation, sleep disturbance, depression, convulsions, dilated pupils, dry mouth, constipation, abnormally high temperature, possible loss of consciousness, coma. The maximum permissible daily dose of chlorphenamine maleate for adults and children over 15 years of age is 24 mg, for elderly patients - 12 mg. Symptoms of paracetamol overdose: nausea, vomiting, loss of appetite, pallor, abdominal pain. These symptoms appear mainly in the first 24 hours. An overdose, starting with 10 g of paracetamol at one time for an adult and 150 mg per 1 kg of weight at one time for a child, leads to liver cytolysis, which can lead to complete and irreversible liver necrosis, expressed in liver failure, metabolic acidosis, encephalopathy up to coma and death. At the same time, there is an increase in the level of liver transaminases, lactate dehydrogenase, bilirubin and a decrease in the level of prothrombin, which can manifest itself 12-48 hours after administration. An overdose of ascorbic acid increases the risk of hemolysis and the formation of kidney stones. After a single dose of 3 g, diarrhea and gastrointestinal symptoms such as nausea or gastritis almost always develop after a single dose of 10 g. The literature describes isolated cases of acute and chronic overdose of ascorbic acid (taking more than 4 g/day) in patients with glucose-6-phosphate dehydrogenase deficiency. In DIC, an overdose of ascorbic acid can lead to a significant increase in oxalate levels in the blood serum and urine. Emergency care: Immediately transport the patient to the hospital; collecting a blood test in a test tube to determine the initial concentration of paracetamol in plasma; rapid elimination of the drug taken by gastric lavage; Treatment of paracetamol overdose usually involves administering the antidote N-acetylcysteine intravenously or orally as early as possible, within the first ten hours of administration if possible; symptomatic treatment.
Side effect
Minimizing the risk of adverse reactions is facilitated by compliance with the recommended doses and duration of drug use. Side effects may occur with varying frequency: Associated with the presence of paracetamol. Isolated rare cases of immediate hypersensitivity reactions have been observed: anaphylactic shock, Quincke's edema, erythema, urticaria, skin rash. If these manifestations occur, you should immediately stop taking this medicine and other medicines containing paracetamol. In very rare cases, thrombocytopenia, leukopenia and neutropenia have been observed. Associated with the presence of chlorphenamine maleate, the pharmacological characteristics of chlorphenamine underlie side effects of varying degrees of intensity, which are associated or not associated with the dosage of Antigrippin. From the side of the autonomic nervous system. drowsiness, more pronounced at the beginning of treatment; orthostatic hypotension; anticholinergic effect: dry mucous membranes, constipation, impaired accommodation, dilated pupils, increased intraocular pressure, rapid heartbeat (arrhythmia is also possible), urinary disorder (dysuria, urinary retention); • imbalance, dizziness, decreased memory or concentration, more common in older people; loss of coordination of movements, tremors; confusion, hallucinations. Hypersensitivity reactions: erythema, eczema, severe itching, purpura; less commonly, Quincke's edema; anaphylactic shock. From the hematopoietic organs: leukopenia, neutropenia; thrombocytopenia; hemolytic anemia. Other (frequency unknown): hepatitis, jaundice, muscle twitching, muscle weakness, chest tightness, fatigue. Neurological anticholinergic effects and paradoxical arousal (eg, hyperactivity, restlessness, nervousness) are more common in children and elderly patients. Associated with the presence of ascorbic acid Allergic reactions and irritation of the gastrointestinal mucosa are possible. Daily doses of vitamin C greater than 600 mg have a diuretic effect. With long-term use of large doses of vitamin C, flushing or redness of the skin, nausea, vomiting, diarrhea, ulceration of the gastrointestinal mucosa, increased excitability, fatigue, sleep disturbance, headache, and damage to the insular apparatus of the pancreas may occur. When using large doses of vitamin C (usually a daily dose of more than 1 g), cases of hyperoxaluria and the appearance of oxalate stones, and hemolysis in patients with glucose-6-phosphate dehydrogenase deficiency have been reported. The use of ascorbic acid in therapeutic doses may distort the results of tests to determine glycosuria, uric acid and creatinine levels, as well as the results of various laboratory tests (blood glucose, bilirubin, transaminase activity, lactate dehydrogenase). A dose of 1 g may produce false-negative results in stool occult blood tests. If any adverse reactions occur, including those not listed in these instructions, you should consult a doctor.
Storage conditions
At a temperature not exceeding 25 °C. Keep out of the reach of children!
Manufacturer
Natur Product Europe B.V. (Netherlands), Natur Product Pharma Sp z o.o. (Poland) by order and under the control of Natur Product Europe B.V. (Netherlands) (Antigrippin effervescent tablets, Antigrippin effervescent tablets for children).
Natur Product Pharma Sp z o.o. (Poland) by order and under the control of Natur Product Europe B.V. (Netherlands) (Antigrippin effervescent tablets with raspberry, grapefruit flavors, Antigrippin powder for the preparation of a solution for oral administration, honey-lemon, chamomile).