What are bacteriophages?
It is well known that bacteriophages are able to adapt to new conditions thanks to mutations, but among the signs of “living” they have only the ability to reproduce and transmit hereditary information to descendants. It is these properties that have allowed humans to use them as an alternative to antibiotics to fight infections and destroy pathogenic bacteria.
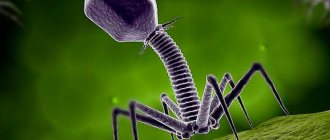
Bacteriophages are viruses, tiny natural structures similar to molecular crystals. But, unlike most viruses known to mankind, they do not infect higher organisms (for example, humans), but only lower ones - single-celled ones; it is not for nothing that “bacteriophage” is literally translated as “eater of bacteria”. Bacteriophages are so simple that they cannot even reproduce on their own - for this, like other viruses, they need a “foreign” living cell.
Phage cocktails
A new round of interest in phage therapy has occurred in recent years. The fact is that antibiotics have also not become a panacea for the treatment of bacterial infections: these days, the development of new drugs has not kept pace with the increase in the number of bacteria with acquired resistance to existing antibiotics. Already today, in hospitals in England, about 40% of staphylococcal infections are caused by such strains, and in the United States, about 90 thousand patients die annually from hospital infections caused by drug-resistant bacteria. When recalculated for the world's population, this number is 3-5 million deaths per year!
WHO warns that the world will soon enter a “post-antibiotic” era, when there will be no treatment for common bacterial infections. And against this background, phage therapy looks like a very promising direction, the development of which can lead to the creation of effective personalized methods for treating diseases. For this, there is both the necessary knowledge about phages and the mechanisms of their interaction with bacterial cells, as well as technologies for working with viral agents.
For phage therapy today, only virulent lysis phages are used, mainly “tailed” phages of the order Caudovirales, as well as filamentous phages of the families Leviviridae (with a single-stranded RNA genome) and Inoviridae (with a single-stranded circular DNA genome).
As discussed above, the activity spectra of phages are usually very narrow and limited to one or a few closely related bacterial species. On the one hand, such narrow specificity is good for therapy, since it allows you to eliminate a specific microorganism without disturbing the entire bacterial community of the human body. On the other hand, if emergency treatment is necessary (when there is no time to identify a specific bacterium causing the development of a pathogenic process in a wound or on a burned surface), it is necessary to have a drug that affects several types of bacteria, possible causative agents of infection. To solve this problem, phage cocktails are usually used - preparations containing several phages that differ in specificity.
This approach was also used by d'Herelle. D'Herelle's cocktail, which he brought from Paris back in 1930, is still one of the main phage preparations: it forms the basis of the Georgian pyophage and the Russian intestifage. In Tbilisi, based on phage cocktails, drugs were developed for the treatment of gastrointestinal diseases and purulent wounds for mass use in the event of epidemics or military operations. The results of army trials and a wide experiment on the prevention of childhood gastrointestinal disorders conducted in Tbilisi showed the good effectiveness of such drugs.
Phage cocktails are produced in a standardized manner and target bacterial communities commonly found in specific diseases. Of course, more effective cocktails are obtained when their components are matched to the bacterial community of a particular patient. To obtain such a cocktail, it is necessary to test the patient’s bacteria for sensitivity to phages from the collection in order to select the most effective phage strains. If the required phages are not in the collection, bacteria-specific phages are searched for in natural substrates.
In general, the search for bacteriophages is quite simple: a bacterial culture is exposed to samples from various sources: water bodies, soil, sewage, etc. If the bacteria die, they are separated from the solution by centrifugation, and the remaining solution is tested for activity. The phage is then propagated by growing on an appropriate bacterial culture. Moreover, phages can be lyophilized (vacuum dried) and directly used in capsules. In this form, the drug remains stable for 14 months at temperatures up to 55 °C.
What does a bacteriophage consist of?
A typical phage consists of a “head” with a tightly packed genetic program consisting of nucleic acids (DNA or RNA), and a “tail” with which it “injects” its genes into the bacterial cell. The infected bacterium begins, using its own intracellular systems and resources, to synthesize proteins and nucleic acids necessary for the assembly of new viral particles. Mature phages go out in search of new prey, and the “parent” bacterial cell dies.
Thanks to recent research, it has become clear that bacteriophages play an important role in maintaining the global “microbial balance” in the biosphere: every two days they destroy half of the world’s bacterial population and thereby prevent these rapidly reproducing organisms from covering the earth’s surface with a thick layer.
Bacteriophages appear wherever bacteria live: on land and in the oceans, in soil and water, in plants and animals. Even the human gastrointestinal tract contains about 1012 bacteriophages - an order of magnitude more than the stars in our Galaxy! And although the size of phage particles does not exceed 0.0001 mm, the biomass of phages on the planet reaches a fantastic figure - 1 billion tons. Therefore, these invisible but omnipresent creatures are sometimes called the “dark matter” of the biosphere.
"Counters" of bacteria
Bacteriophages serve not only as a versatile therapeutic and “disinfectant” agent, but also as a convenient and accurate analytical tool for a microbiologist. For example, due to their high specificity, they are natural analytical reagents for identifying bacteria of a certain type and strain.
In the simplest version of such a study, various diagnostic bacteriophages are added dropwise to a Petri dish with a nutrient medium seeded with a bacterial culture. If the bacterium turns out to be sensitive to the phage, then in this place of the bacterial “lawn” a “plaque” is formed - a transparent area with killed and lysed bacterial cells.
By analyzing the reproduction of phages in the presence of target bacteria, it is possible to quantify the number of the latter. Since the number of phage particles in a solution will increase in proportion to the number of bacterial cells contained in it, to estimate the number of bacteria it is enough to determine the titer of the bacteriophage.
The specificity and sensitivity of such an analytical reaction are quite high, and the procedures themselves are simple to perform and do not require complex equipment. It is important that diagnostic systems based on bacteriophages signal the presence of a living pathogen, while other methods, such as PCR and immunoanalytical methods, only indicate the presence of biopolymers belonging to this bacterium. This type of diagnostic methods is especially convenient for use in environmental studies, as well as in the food industry and agriculture.
reference species are used to identify and quantify different strains of microorganisms
phages.
Very fast, almost real-time analytical systems can be created based on genetically modified bacteriophages, which, when they enter a bacterial cell, trigger the synthesis of reporter fluorescent (or luminescent) proteins, such as luciferase
. When the necessary substrates are added to such a medium, a luminescent signal will appear in it, the value of which corresponds to the content of bacteria in the sample. Such “light-labeled” phages were developed to detect dangerous pathogens such as plague, anthrax, tuberculosis, and plant infections.
It is likely that with the help of modified phages it will be possible to solve a long-standing problem of global importance - to develop cheap and fast methods for detecting tuberculosis pathogens at an early stage of the disease. This task is very difficult, since the mycobacteria that cause tuberculosis are characterized by extremely slow growth when cultivated in laboratory conditions. Therefore, diagnosing the disease using traditional methods can be delayed for up to several weeks.
Phage technology makes this task easier. Its essence is that bacteriophage D29, which is capable of infecting a wide range of mycobacteria, is added to the blood samples being analyzed. The bacteriophages are then separated and the sample is mixed with a fast-growing, non-pathogenic culture of mycobacteria that is also sensitive to this bacteriophage. If the blood initially contained mycobacteria that were infected with phages, then the production of bacteriophage will also be observed in the new culture. In this way, single mycobacterial cells can be detected, and the diagnostic process itself is reduced from 2–3 weeks to 2–5 days (Swift & Rees, 2016).
Advantages of bacteriophages
| Bacteriophages – antibacterial agents and natural antiseptics | Safe and non-toxic, no side effects, used in newborns, pregnant and lactating women |
| The action of bacteriophages does not affect the beneficial microflora of the body, unlike antibiotics | Bacteriophages are compatible with all medications. The use of bacteriophages does not limit the use of other drugs and does not affect their effectiveness |
| Affects only pathogenic bacteria that are sensitive to them and cause infectious disease, destroying them from the inside | Bacteriophages are eliminated from the body naturally |
Magazine for pharmacy business professionals
Bacteriophages are one of the most mysterious drugs in the pharmacy range. Now they are experiencing a rebirth - both in our country and abroad, interest in them has arisen again; not only revisions of old results, but also a lot of new publications appear in journals. What can you tell a buyer who is interested in these particular products?
Milestones of history
Scientists discovered traces of the vital activity of bacteriophages long before virology arose. British bacteriologist Ernest Hankin noticed back in 1896 that a certain agent capable of passing through a very thin porcelain filter kills the causative agent of cholera. The development of science in a new direction was prevented by the First World War, since funding was redirected to the developers of combat gases, explosives and other more relevant things.
Two researchers are considered the discoverers of bacteriophages: the British bacteriologist Frederick Twort, who described a strange filtering agent in 1915, and the Canadian microbiologist of French origin Felix D'Herelle, who, independently of Twort, reported exactly the same discovery in 1917.
Abroad, microbiologists were primarily interested in bacteriophages, so their findings had no practical significance. For a very long time, for example, a phage was considered not a virus, but an enzyme. In the USSR, work was initially carried out by doctors; in Georgia, in 1923, the future All-Union Center for Phage Therapy was created, which at the peak of its heyday collected a collection of more than 3 thousand samples. However, then, both here and abroad, the work of phage therapy was practically destroyed by the appearance of antibiotics. Their massive distribution after World War II created the impression that a panacea against infections had been invented and that other areas of research were dead ends.
Scientists remembered bacteriophages when they were faced with the problem of antibiotic resistance. Viruses again became the object of experiments; they were seen as a real alternative to morally and sometimes even physically outdated antimicrobial drugs.
Natural enemy
A bacteriophage, as the name suggests, is a virus that eats bacteria. In fact, this is a “bacterial flu”, only deaths occur much more often than in the human version.
Phages are much more diverse than any other known viruses; they cannot be said to look the same. Most often, as an illustration, an image of viruses of group A according to the Bradley classification is used, which is also group V according to the Tikhonenko classification. Such viruses are the most complex; they have a head and an appendage with many additional elements (Fig. 1). A total of six groups are distinguished, only in one of them the phage genome is represented by single-stranded RNA, in all other cases it is single- or double-stranded DNA.
Bacteriophages cannot be called giants, but in terms of their size they are rather large and occupy an intermediate position between the largest tobacco mosaic viruses and human immunodeficiency virus type 1 (Fig. 2).
Group A (V) viruses introduce their DNA into the target cell very beautifully, the process is somewhat reminiscent of landing a research module on the surface of the Moon or Mars - in the same way, it first “sits” on the “supports” and then presses against the membrane of its bacteria basal plate, “drills” into the victim’s shell and injects its genome into the cytoplasm. After this, the bacteriophage can be considered dead, if this can be said about viruses at all.
Fast or painful?
Based on their mechanism of action, all bacteriophages can be divided into two large groups. The first is lytic, or virulent phages, which in 100% of cases kill the infected cell immediately. In the case shown in Figure 3, the virus immediately destroys the bacterial genome, using it exclusively for the purpose of its own replication. After producing the maximum possible number of new viral particles, the microbe either dies immediately at the moment of their release, simply breaking into pieces, or dies in the near future due to the fact that all critical internal mechanisms are physically destroyed.
The second group is lysogenic, or temperate phages. Their genome is integrated into the genome of the host and can exist there in stealth mode for a long time, passed on from generation to generation. This “time bomb” is activated under the influence of various unfavorable external factors such as nutritional deficiency. Single phages are able to combine both options, budding and not immediately destroying the bacterial cell, only using part of its membrane as a shell, while simultaneously leaving a “bookmark” in its genome. One microbe can be attacked by several phages with different interaction mechanisms at once.
Advantages and disadvantages
The undoubted advantages of phages include their targeting; they specialize in specific types of bacteria, so it is possible to select those viruses that destroy only pathogenic flora, in contrast to antibiotics that “hit areas” without distinguishing whether it is your own or someone else’s under fire.
For the same reason, phages have either fewer or no side effects. The story with resistance is similar; if it develops, it is only in a specific target bacterium. With the help of genetic engineering, it is possible to reprogram the virus and set it against some other pathogen that is not yet familiar with the virus.
An important factor in favor of bacteriophages can be considered the cheapness and ease of their production, especially in comparison with new promising antibiotics. Indeed, the result is no less, and sometimes even more effective, alternative with much less investment.
There are also disadvantages, and they are closely related to the viral nature of phages. First of all, they are difficult to store and transport; they are very demanding in terms of maintenance conditions. In addition, phages are only capable of attacking free-floating bacteria; no one will allow them inside human cells. The aggressive environment of the stomach destroys many viruses, without distinguishing who came in peace and who did not. And most importantly, to achieve the maximum effect of therapy, the causative bacterium must be phagotyped, that is, isolated and proven in the laboratory that this particular virus can be used against it.
Nevertheless, phages remain a promising area of development, especially after the discovery of Canadian biologist Joseph Bondy-Denomy, working at the University of California. He discovered a new virus that specifically destroyed Pseudomonas aeruginosa, to which resistance had not developed. Studying the phenomenon, the scientist found out that the DNA of this phage is protected by a protein coat, which the bacteria’s immunity cannot cope with. If such a “cover” is put on all other DNA and RNA of bacteriophages, they can become an even more formidable weapon for their natural enemies. The main thing is not to switch to a person.
Data
In the USSR, the medical use of bacteriophages was actively studied, but the publications were either in Russian or Georgian, so world science did not know about them and is surprised to study and quote them in our time.
Work on bacteriophages was awarded the Nobel Prize. In 1969, it was awarded to American researchers Max Delbrück, Alfred Hershey and Salvador Luria for their discoveries concerning the replication mechanism and genetic structure of viruses.
The following types of bacteriophages are registered in the GRLS and are circulating on the Russian market: typhoid, dysentery, against Klebsiella and separately - against Klebsiella pneumonia, against Escherichia coli, Proteus, Salmonella, staphylococcal and against Pseudomonas aeruginosa. The most commonly used drug in outpatient practice is staphylococcal phage.
1. Keen EC Phage Therapy: Concept to Cure // Frontiers in Microbiology. 2012. 3: 238. doi: 10.3389/fmicb.2012.00238. 2. Keen EC Tradeoffs in bacteriophage life histories // Bacteriophage. 2014. 4 (1): e28365. doi: 10.4161/bact.28365. 3. Kutter Elizabeth et al. (2010). Phage Therapy in Clinical Practice: Treatment of Human Infections // Current Pharmaceutical Biotechnology. 11 (1): 69–86. doi: 10.2174/138920110790725401. 4. McGrath S. and van Sinderen D. (editors). Bacteriophage: Genetics and Molecular Biology (1st ed.). Caister Academic Press. 2007. ISBN 978-1-904455-14-1. 5. Stineke van Houte et al. The diversity-generating benefits of a prokaryotic adaptive immune system // Nature. 2021. DOI: 10.1038/nature17436. 6. Pozdeev O.K., Fedorova E.R., Valeeva Yu.V. Microbiology. Bacteriophages/Teaching and methodological manual for students of medical universities. Kazan, 2012.
Alexey Vodovozov
Magazine "Russian Pharmacies" No. 1-2, 2020
Other articles you may like:
Details
Congratulations to our colleagues!
At the end of December, everyone has a very busy schedule and one big problem: you need to have time to choose and buy gifts for friends and family.
And managers should also think about the team. How to please and… Read more
Details
For a winter fairy tale
The coronavirus leaves no chance for foreign travel this year either, and the low rate of vaccination in Russia makes one wonder whether it is really necessary to fly or travel somewhere.
But if there is no doubt,... Read more
Details
Bright future
The fight against pigmentation is not an easy task, but achieving victory is quite possible.
The result will depend on the cause of the spots, their “age” and the method of therapy. More details
Details
Strengthen positions
Summer is over, and it may seem that your hair has rested and gained health.
But in fact, instead of strength and brilliance, they often acquire weakness and fragility. The main reason is a fair amount of ultra… Read more
Details
The night Watch
Night creams have always caused mixed reactions.
Some criticize such products, arguing that the skin should rest in the dark, while others strive to use all ... Read more
Details
Replenishment for the bookshelf
Books on medical and related topics are published with enviable regularity. Publishers are actively translating foreign bestsellers, and domestic doctors and scientists are not lagging behind. Read more
Application of bacteriophages
Immediately after the discovery of bacteriophages, drugs based on them began to be used to combat human infectious diseases. However, as a result of the invention of antibiotics and lack of knowledge about bacteriophages, their therapeutic potential was not realized.
Half a century later, molecular biologists became interested in bacteriophages. They found that these simple “nanodevices” with short genetic programs are convenient objects for experimental studies to study the structure and operation of the genome. Further study of phages and the mechanisms by which bacteria protect themselves from enemies has revealed to science one of the most effective genome editing tools - CRISPR-CAS, based on the “bacterial immunity” system.
Phages have found application in various areas of human activity, including bio- and nanotechnology. For example, as simple systems for producing proteins with given properties or as a basis for creating materials with a given architecture in catalytic chemistry.
As “smart” molecular devices, they are used to transport drugs in the body and as diagnostic sensors, for example, to detect pathogenic bacteria in food. Phage preparations are used for disinfection in agriculture and the food industry. This increases the environmental friendliness of the products.
But still, medicine, like a century ago, remains the main area of application of these bacterial enemies. With the increase in drug resistance of bacteria to chemical antibiotics, the importance of phage therapy for the prevention and treatment of human infectious diseases has increased.
Phage antibiotics
Phages do not need to be used directly for therapeutic purposes. Over millions of years of evolution, bacteriophages have developed an arsenal of specific proteins - tools for recognizing target microorganisms and manipulating the biopolymers of the victim, on the basis of which antibacterial drugs can be created. The most promising proteins of this type are endolysin enzymes, which phages use to destroy the cell wall when leaving the bacterium. These substances themselves are powerful antibacterial agents that are non-toxic to humans. The effectiveness and direction of their action can be increased by changing their addressing structures - proteins that specifically bind to certain bacteria.
Most bacteria are divided according to the structure of their cell wall into gram-positive, whose membrane is covered with a very thick layer of peptidoglycan, and gram-negative, in which a layer of peptidoglycan is located between two membranes. The use of natural endolysins is especially effective in the case of gram-positive bacteria (staphylococci, streptococci, etc.), since their peptidoglycan layer is located on the outside. Gram-negative bacteria (Pseudomonas aeruginosa, Salmonella, Escherichia coli, etc.) are a less accessible target, since the enzyme must penetrate the outer bacterial membrane to reach the inner peptidoglycan layer.
To overcome this problem, so-called artilisins were created - modified versions of natural endolysins containing polycationic or amphipathic peptides that destabilize the outer membrane and ensure the delivery of endolysin directly to the peptidoglycan layer. Artilisins have high bactericidal activity and have already shown their effectiveness in the treatment of otitis media in dogs (Briers et al., 2014).
An example of a modified endolysin that selectively acts on certain bacteria is the drug P128 from the Canadian company GangaGen Inc.
. It is a biologically active fragment of endolysin combined with lysostaphin, a targeting protein molecule that binds to the surface of staphylococcal cells. The resulting chimeric protein has high activity against various strains of staphylococcus, including those with multidrug resistance.
Modern history
To date, specialists from Tbilisi and the specialized center of the Institute of Immunology and Experimental Therapy named after. L. Hirschfeld (Wroclaw, Poland), where bacteriophage preparations for testing are produced in small quantities.
Polish researchers initially focused on personalized therapy. They used phage therapy to experimentally treat patients with chronic diseases that did not respond to antibiotics. Thousands of patients have already passed through the center, many of whom were completely cured.
The results of these clinical trials have proven the high effectiveness of phages in the treatment of infectious pulmonary diseases: a single intranasal administration of the drug is sufficient to suppress infection in the throat, nose and lungs. Phages are no less effective in eliminating pathogenic bacteria from the gastrointestinal tract. The high efficiency of bacteriophages has also been demonstrated in almost all cases of pyogenic diabetic foot ulcers, lung diseases, mastitis, and urogenital infections. The list of such diseases goes on, but it is important to note that none of the trials observed any side effects caused by bacteriophages.
As specific agents that destroy bacteria, bacteriophages today are used in the treatment of diseases not only in humans, but also in animals, as well as for plant protection and food preservation. Thus, in 2006, the FDA approved the use of bacteriophage cocktails for processing meat and other agricultural products. In this case, phages received the status of food additives. They have also been approved for use as a disinfectant. Phage preparations (in the form of aerosols) have been successfully tested in experiments to protect poultry on large farms, as well as in fish farms
In England, phage preparations were successfully tested for the treatment of chronic otitis media, a difficult-to-treat disease due to the formation of so-called bacterial biofilms - drug-resistant microbial films. In France, the cradle of phage therapy, research in this area is now almost non-existent, although until recently the Pasteur Institute made phage cocktails to order.
Today, phage preparations are produced on an industrial scale by the Russian company Microgen. Similar medications can be bought in pharmacies in Russia, Belarus and Ukraine. Phage preparations produced by Microgen and the Tbilisi Center for the treatment of burn infections were successfully tested in Belgium.
However, the use of bacteriophages in therapy is still not officially permitted in most countries: this applies to both the FDA, the American Food and Drug Administration, and similar European agencies. In the European Union, phages are used to treat patients only at the aforementioned Polish Institute of Immunology and Experimental Therapy.
Therefore, treatment of interested patients is carried out in the mode of medical tourism. (California, USA) refers patients from different countries suffering from chronic diseases caused by drug-resistant bacteria either to the Phage Therapy Center in Tbilisi or to its clinic in Mexico.