Synonyms: EO
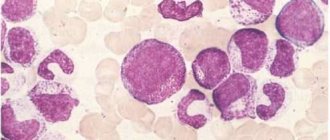
Eosinophils consist of a bilobed nucleus and three types of eosinophil granules (lipid bodies, eosinophils, small and primary). They contain protein structures with the help of which foreign cells are neutralized. Eosinophils are quite large cells: their diameter reaches 20 microns.
Eosinophils got their name due to the fact that when stained according to Romanovsky, they are easily stained with eosin (an acidic dye), while other types of dyes have no effect on them. Eosin itself was invented in 1873 by the German scientist G. Caro and, due to its bright pink color, was named after the ancient Greek goddess of the dawn Eos (in the German version the name sounds like “eosin”).
What are eosinophils?
The content of the article
Eosinophils are a type of granular leukocytes or granulocytes. Their cells include a bipartite nucleus and three types of eosinophilic granules - lipid bodies. They contain protein structures that neutralize foreign bodies and substances. Eosinophils are large cells with a diameter of up to 20 microns.
Eosinophils got their name because when stained according to Romanovsky, they are easily stained with eosin dye. This dye was invented in 1873 by the German scientist G. Caro and, because of its bright pink color, was named after the ancient Greek goddess of the dawn Eos - in the German version “eosin”.
Eosinophils are formed in the red bone marrow within 3-4 days. After this, they enter the bloodstream, accumulating in the mucous membranes of the intestines, respiratory tract and capillaries. There they live and function for 10-14 days. In peripheral blood the concentration of eosinophils is insignificant. There are about 200 times more of them in the bone marrow and other tissues.
Role and functions in the body
Eosinophils, as a type of white blood cell, are responsible for protection against foreign agents, which can be microorganisms, chemicals, and toxins. Eosinophils are often called scavenger cells, which have a special task. They are considered the most sensitive to parasitic infections and pathological bacteria. They usually begin their work after lymphocytes and neutrophils. Their option in this case is to dissolve the remains of pathogenic microorganisms.
These cells also participate in the “antigen-antibody” reaction, due to which they control the release of histamine, better known as a substance that causes allergies. Thanks to this, eosinophils help reduce the aggressiveness of the immune system's response to foreign proteins. Such blood cells can also penetrate vascular walls and move through tissues to the site of damage or inflammation.
Among the lesser known areas of responsibility of eosinophils is the prevention of thrombosis. However, they also have a downside. These blood cells can become dangerous, for example, when they are associated with pathological changes. This happens with Loeffler's disease. This is an allergic disease, when the number of eosinophils in the blood increases, and infiltrates form in the lungs, which, however, quickly disappear.
Vital interest. Six naive questions about tests Read more
Indications for analysis
A complete blood test with a count of eosinophils is prescribed for most people who visit the clinic.
The range of indications is very wide, but in modern clinical practice the initial eosinophil count is most often used:
- as a biomarker for assessing the effectiveness of drugs prescribed to patients with bronchial asthma;
- upon confirmation of a preliminary diagnosis;
- checking the effectiveness of the prescribed therapeutic course;
- assessing a person’s health status during medical examinations or medical examinations;
- in the postoperative period;
- if the development of infectious, oncological and autoimmune diseases is suspected.
FAQ
h25,0,0,0,0—>
Question: Is it possible to get rid of eosinopenia with herbs? If yes, which ones?
p, blockquote21,0,0,0,0—>
Answer: It is not the deviation in the UAC itself that needs to be treated, but its causes. It is not a fact that the problem is caused by a depressed state of immunity. It is better not to self-medicate. There is a risk of missing a dangerous disease and even worsening its course. If a person knows for sure that disorders are caused by stressful situations or a lack of nutrients in the diet, high loads, it makes sense to resort to general strengthening measures. A balanced diet, moderate physical activity, hardening, and frequent walking help normalize the functioning of the defenses. When you really want to resort to herbal medicine, you can use herbs with adaptogenic properties. These include Echinacea purpurea, Rhodiola rosea, Eleutherococcus and ginseng. Their extracts can be purchased at the pharmacy in ready-made form.
p, blockquote22,0,0,0,0—>
- Why are eosinophils in the blood low, what does this mean?
Question: How to prepare for the analysis in order to exclude a false positive result? I’m taking the UAC test for the 3rd time, the previous 2 times the doctor doubted that the result was correct.
p, blockquote23,0,0,0,0—>
Answer: 3 days before the test, you need to stop taking medications and expose yourself to significant physical activity. It is advisable to give up fatty foods and moderate the amount of protein foods in the diet. It is worth excluding alcoholic beverages and various “harmful” foods (fast food, processed foods, chips, crackers). The last meal should take place 8–12 hours before the collection of biomaterial. It is forbidden to consume any food or drinks other than pure water (you will also have to skip morning coffee). It is better not to smoke directly on the day of the test. It is worth limiting yourself from stress, overheating or freezing on the way to a medical facility.
p, blockquote24,0,0,1,0—>
Question: Is it possible to take the OAC during menstruation? How can they affect performance?
p, blockquote25,0,0,0,0—>
Answer: It is not worth taking a test during menstruation unless absolutely necessary. During this period, any of the indicators may go beyond the normal range, which is not associated with the disease. Doctors recommend refraining from any laboratory tests 3 days before, immediately during bleeding, and 2-3 days after. However, regarding eosinophils, there is another version. It is believed that the importance of these blood cells may reduce progesterone. If it is eosinophils that cause doubts, it is worth getting tested after menstruation, but before ovulation (7-13 days of the cycle).
p, blockquote26,0,0,0,0—>
Question: Is it possible to reduce the level of eosinophils with drugs used for allergies to critical levels?
- Why are eosinophils elevated in the blood, what does this mean?
p, blockquote27,0,0,0,0—>
Answer: Antihistamines have little effect on the blood picture if they are used correctly and the recommended doses are followed. It is important to observe the duration of the course, take breaks or change medications on time. Significant deviations in the blood picture can be caused by hormonal drugs, so you need to be careful with them and use them only as prescribed by a doctor.
p, blockquote28,0,0,0,0—>
Question: Will herbal remedies from the group of immunostimulants (for example, Immunal or Immupret) help increase the level of eosinophils?
p, blockquote29,0,0,0,0—>
Answer: They will help if the deviation is caused by an infectious disease, exhaustion of the body, or stress. If the nature of the disorders is purulent processes, malignant or hereditary pathologies, immune stimulants will not help, and in diseases of the bone marrow or endocrine glands they can even cause harm.
p, blockquote30,0,0,0,0—>
Norms of eosinophils in the blood
| Age | Normal level of eosinophils in the blood |
| Children under 5 years old | 0,5 – 7% |
| Children 5 – 14 years old | 1 – 5% |
| Adults | 0,5 – 5% |
In women, in the first days of menstruation, the level of eosinophils is slightly higher than normal, and after ovulation their number is slightly lower than normal.
An increased level of eosinophils in the blood is called eosinophilia.
Consequences of deviation from the norm
A change in the normal level of eosinophils in the blood is not a criterion by which one can get an idea of possible pathological changes in the body. Simply put, the level of eosinophils in the blood itself means little - you need to pay attention to the disease that caused its change. Therefore, the consequences can be very diverse - from completely insignificant (as in the case of physical activity) to deadly (for example, in the case of oncology).
Eosinophilia itself, in rare cases, can lead to damage to tissues in which the greatest accumulation of eosinophils is observed. The mechanism of such damage has not been fully studied to date, but it has been established that eosinophils exhibit the most severe damaging effect in conditions such as eosinophilic fibroplastic endocarditis and idiopathic hypereosinophilic syndrome. The nature of the damage directly depends on the duration of eosinophilia and the severity of eosinophilic tissue infiltration.
Stages of eosinophilia
| Stage | Stage level | The number of eosinophils in the blood from the total number of leukocytes |
| First | Lightweight | no more than 10% |
| Second | Average | 10% – 15% |
| Third | Heavy | over 15% |
Blood test for eosinophils
To determine the level of eosinophils, a general blood test is used, which is familiar to all of us since childhood. Eosinophils by themselves do not give an accurate idea of the nature of pathological changes in the body, however, in combination with other values of the leukocyte formula, they allow a specialist to judge the nature of the pathology. In the analysis form, eosinophils are designated as EO. Their content is indicated as a percentage of the total number of leukocytes. A formula is also used to calculate the absolute number of eosinophils in peripheral blood and it looks like this: number of leukocytes * 10 to the 9th power. For a general analysis, blood is most often taken from a finger prick, but venous blood is also suitable.
Figure 1. Causes of eosinophilia (increased number of eosinophils in the blood). Image: kavusta/Depositphotos
Causes of increased levels of eosinophils in the blood
An increase in eosinophils or eosinophilia is observed in the following diseases and conditions:
- diseases and conditions accompanied by allergic processes in the body - bloating, bronchial asthma, urticaria, Quincke's edema, serum sickness, drug sickness, etc.;
- parasitic diseases – ascariasis, giardiasis, toxocariasis, trichinosis, opisthorchiasis, echinococcosis, malaria, etc.;
- concomitant tissue diseases and systemic vasculitis - rheumatoid arthritis, mesothelial periarteritis, scleroderma, systemic lupus erythematosus, etc.;
- dermatological diseases - dermatitis, eczema, skin crocus, pemphigus, etc.;
- some infectious diseases - tuberculosis, scarlet fever, syphilis;
- blood diseases accompanied by the spread of one or more hematopoietic bacteria - chronic myeloid leukemia, erythremia, lymphogranulomatosis;
- taking sulfonamides, antibiotics and adrenocorticotropic hormones.
Long-term (more than six months) high eosinophilia of unknown etiology is called hypereosinophilic syndrome. The level of eosinophils in the blood exceeds 15%. This pathology is very dangerous, it causes damage to internal organs - heart, kidneys, bone marrow, lungs, etc.
If the content of monocytes and eosinophils in the blood is increased, this may indicate an infectious process in the body, a blood disease, or an early stage of cancer.
Sometimes the number of monocytes increases after recovery from various diseases.
Tasks of eosinophils
The main task of eosinophils is the elimination of foreign harmful agents.
Their destruction occurs at the extracellular level; their capabilities also include the elimination of fairly large organisms. The effect begins when the contents of intracellular granules are released. Compared to neutrophils, the ability for phagocytosis in the agents we are considering is less, but it is still present. This is not their main task, but they can destroy and absorb germs. Let us list the main functions of eosinophilic granulocytes:
- They have a toxic effect on helminths.
- Eliminate the effect of biologically active substances that cause allergies.
- They help eliminate the consequences of the activity of bioactive substances that were produced by mast cells and basophils. The latter are the main causative agents of an allergic reaction. They also influence the development of severe forms of the disease - Quincke's edema and anaphylactic shock.
- Develop a highly sensitive reaction.
- Awaken activity to kill bacteria.
- Eliminate foreign cells by absorbing them.
Eosinophils fight allergens, leading to stabilization of the child or adult's condition
How to reduce the number of eosinophils in the blood?
First of all, it is important to eliminate the cause of eosinophilia, that is, the disease that caused a change in the leukocyte formula.
Possible treatment options:
- antiallergic therapy - taking antihistamines: chloropyramine, cetirizine, levocetirizine, desloratadine and loratadine, etc.;
- antibacterial therapy - taking antibiotics: amoxicillin, ceftriaxone, azithromycin, erythromycin, etc.;
- anti-inflammatory therapy - taking drugs for inflammatory processes, for example glucocorticosteroids - prednisolone;
- chemotherapy – to eliminate malignant tumors;
- hormone therapy;
- anthelmintic therapy - taking albendazole, levamisole, bephenium hydroxynaphthoate, piperazine, tetrachlorethylene, mebendazole. The choice of medication depends on the type of parasite and the stage of infestation.
Additionally, the following measures can be taken:
- Normalize your lifestyle. It is important to avoid frequent drinking of alcohol and give up cigarettes.
- Avoid chronic intoxication. In people working in hazardous industries or living in environmentally unfavorable regions, the number of eosinophils in the blood increases as a result of constant chemical intoxication.
- Stick to a healthy diet. You should not overuse spicy, smoked, canned and fatty foods. To increase the level of eosinophils, you should limit the amount of meat, poultry and fish in your diet, and consume mainly their low-fat varieties. The menu should include yogurt, cheeses, vegetables, fruits, beans, bran and whole grain bread.
Etiology
An increase in eosinophils in a child has the following pathological causes:
- allergic reactions;
- helminthic infestations;
- lack of magnesium;
- immunodeficiency states;
- systemic diseases;
- infectious and viral diseases;
- chronic skin diseases;
- dysfunction of the thyroid gland;
- diseases of the upper respiratory tract, most often pneumonia;
- extensive thermal burns;
- poisoning with toxic substances;
- congenital diseases of the cardiovascular system;
- increased tone of the vagus nerve;
- polycythemia;
- tuberculosis;
- vasculitis;
- Infectious mononucleosis;
- benign tumors;
- oncological processes.
In addition, eosinophils in the baby’s blood can be increased with long-term use of drugs of this type, such as sulfonamides, nitrofurans, hormones and antibiotics.
The reasons that eosinophils are higher than the permissible number can only be established through diagnostic measures, since this process does not have a specific clinical picture.
How to increase the number of eosinophils in the blood?
A decrease in the level of eosinophils in the blood is a sign of weakened immunity. As in the case of eosinophilia, the primary pathology should be identified and eliminated, as well as lifestyle adjustments:
- minimize bad habits;
- ensure an adequate level of physical activity;
- take care of the normal daily routine;
- consume more foods containing vitamins B12, C, D - meat, fish, cottage cheese, rose hips, currants, garlic, or use pharmacy multivitamins.
- It is advisable to exclude foods with a high allergenic potential: milk, soy, wheat, eggs, seafood, peanuts.
Changes in the level of eosinophils in the blood can be caused by both physiological reasons - physical activity, stress, overeating, and various diseases. Sometimes the same condition, for example, an allergy or an infectious process, can cause both a decrease and an increase in the number of eosinophils in the blood and other biological fluids.
IgE, mast cells, basophils and eosinophils
IgE, mast cells, basophils and eosinophils are important elements of allergic inflammation. Allergen-specific IgE, synthesized in response to allergens in the environment and in susceptible individuals, becomes fixed to high-affinity receptors on cell membranes, especially mast cells and basophils. If these receptor-bound IgE molecules aggregate upon repeated exposure to a specific allergen, these mast cells and basophils produce mediators that lead to an allergic response. The main cells attracted to mediator release sites are eosinophils.
IgE
Reagin antibody IgE (immunoglobulin E) has an approximate molecular weight of 190 kDa, does not have transplacental transfer and, unlike other immunoglobulins, does not activate complement through the classical pathway. IgE is heat labile and will not be sensitized after being heated to 56°C for several hours. IgE is mainly known for its ability to bind with high affinity to its specific receptor (R) FcϵRI, found in its full form (α 2 ) on the membranes of mast cells and basophils. Serum IgE concentration is the lowest of the five human immunoglobulin isotypes (0-0.0001 g/L, representing 0.004% of total serum immunoglobulin) and is highly age dependent. Serum IgE concentrations are low in umbilical cord serum (<2 kU/L, <4.8 mg/L) and increase with age until the individual reaches 10–15 years of age. . People with an allergic predisposition experience an earlier and steeper rise. Total serum IgE levels decline from the second to eighth decades of life. According to experts, about 50% of the body's total IgE is found inside the blood vessels. IgE has a half-life of 1 to 5 days in peripheral blood.
B cells initially produce IgM antibodies, but after appropriate stimuli they change the isotype of the antibody produced, maintaining antigen specificity by sharing the same variable (VDJ) region. This “isotype switching” is effective in the sense that it allows one B cell clone to produce antibodies with the same specificity but different effector functions found in the constant portion of the heavy chain. This process consists of splicing and rejoining genomic DNA to map VDJ elements to C-region exons that encode, in the case of IgE, the ϵ-strand that determines the IgE isotype. IgE synthesis requires two types of signals. Signal 1 is provided by the cytokines interleukin (IL)-4 and IL-13, which activate transcription at a specific immunoglobulin locus. The second signal is provided by CD40 ligation on B cells, which in turn activates DNA switch recombination. Both signals are represented by B cells and T cells.
The process begins when the allergen binds to allergen-specific IgM on a B cell, which then processes the allergen. When the B cell then presents fragments of this allergen in the context of MHC class II molecules in the T cell receptor-CD3 complex on a T H 2 cell, the T cell rapidly expresses IL-4 ligand and CD40 (CD40L, CD154), CD40L includes CD40, expressed in the B cell. This interaction results in the expression of B7 on B cells, which binds to CD28 on the T cell, resulting in upregulation of T cell-derived IL-4.
Human mast cells and basophils have been reported to secrete IL-4, IL-13, or both, and also express some CD40L. Thus, these observations suggest that these cells may interact with B cells to provide signals for IgE synthesis or amplification. Since the production of IL-4 or IL-13 by basophils and mast cells depends on the aggregation of Fc-RI through antigen-specific IgE, this mechanism is more attractive for amplification of IgE synthesis. This mechanism does not appear to be allergen specific, but rather produces a polyclonal response. This hypothesis is consistent with the observation that the IgE response in hyper-IgE states is polyclonal.
There are two different IgE receptors: the low-affinity IgE receptor (FcϵRII; CD23), present on B cells, and the high-affinity IgE receptor (FcϵRI). Fc-RI on mast cells and basophils is a tetramer (α2), whereas it is expressed in a trimeric form (2) on antigen-presenting cells such as monocytes, Langerhans cells, and peripheral blood dendritic cells. Human basophil human Fc-RI expression density correlates with serum IgE levels because IgE binding to Fc-RI stabilizes the receptor on the cell surface. Fc-RI-IgE interactions may also promote mast cell survival.
Total IgE levels are affected by age, genetic predisposition, ethnicity, immune status, season and certain diseases. Elevated levels of IgE are found in parasitic diseases such as schistosomiasis and hookworm; infections such as allergic bronchopulmonary aspergillosis and Epstein-Barr mononucleosis virus; skin diseases such as bullous pemphigoid; tumor diseases such as Hodgkin's disease and IgE myeloma; immunodeficiency diseases such as Wiskott-Aldrich syndrome, hyperimmunoglobulinemia E syndrome, thymic hypoplasia (DiGeorge syndrome) and cellular immunodeficiency with immunoglobulins (Nezelof syndrome); and a number of other diseases such as nephrotic syndrome, cystic fibrosis, Kawasaki disease and polyarteritis nodosa. Taken together, such information dictates that measurement of total serum IgE levels is of limited value as a screening test for allergic disease.
Basophils
Basophils are granulocytes that are believed to represent a separate lineage from mast cells, despite the fact that the two cell types share many features such as high-affinity IgE receptor (FcϵRI) expression, metachromatic staining, TH 2 cytokine expression and release histamine. Basophils make up less than 1% of peripheral blood leukocytes, making them one of the least abundant cell lineages in peripheral blood. The number of basophils in peripheral blood is slightly increased (approximately 2 times) in allergic asthma. Basophils have a segmented nucleus with highly condensed chromatin and are usually identified by metachromatic staining with basic dyes such as toluidine blue. Recently, two monospecific antibodies to basophil granules, BB-1 and 2D7, have been developed that allow the unambiguous identification of basophils in tissues and significantly expand our understanding of the role of basophils in allergic diseases and asthma. Basophils express various cytokine receptors (IL-3R, IL-5R, GM-CSFR), chemokine receptors (CCR2, CCR3), complement receptors (CD11b, CD11c, CD35, CD88), prostaglandin receptors (CRTH2) and immunoglobulin Fc receptors ( FcϵRI, FcγRII).
Basophils develop from CD34+ pluripotent stem cells, differentiate and mature in the bone marrow, and then circulate in the periphery. IL-3 is the dominant cytokine that drives basophil differentiation and is sufficient for the differentiation of stem cells into basophils. The general consensus is that basophils are a separate cell lineage from mast cells and are distinct from the common basophil-eosinophil precursor; This belief is supported by the origin of mixed colonies of basophils and eosinophils from individual progenitor cells.
As with mast cells, basophils express a complete and functional Fc-RI receptor (αβ 2 ), the cross-linking of which leads to basophil activation, exocytosis of granules. and release of the intermediary. C3a and C5a can also activate basophils through the complement receptors C3aR and C5aR, respectively. Activation through any of these receptors results in histamine release, eicosanoid synthesis, and IL-4 and IL-13 gene expression. Priming is the ability of molecules that cannot maximally activate basophils on their own to enhance Fc-RI-mediated activation. Mediators with such priming activity include CC chemokines (eotaxins, monocyte chemoattractant protein 3, monocyte chemoattractant protein 4, RANTES), N-formyl-methionyl-leucyl-phenylalanine, IL-3, IL-5, GM-CSF, and histamine releaser. factor. The presence of such mediators at sites of allergen exposure can reduce the threshold for the development of allergic inflammation.
Basophils produce many mediators similar to mast cells, such as histamine, leukotrienes, IL-4 and IL-13. Conversely, the mast cell mediators PGD 2 and IL-5 are not produced by basophils. Basophils mainly generate LTC 4 from newly synthesized eicosanoid mediators. In addition to histamine, basophil granules contain a number of other preformed mediators such as chondroitin sulfate, basic basic protein, and Charcot-Leyden crystalline protein. Typically, basophils contain only small amounts of tryptase; however, there appears to be a lot of variation between individuals regarding basophil tryptase expression. In addition to their role in direct hypersensitivity, basophils may contribute to allergic inflammation through a number of non-classical mechanisms. Basophilic expression of IL-4 and CD40L induces IgE B cell switching in vitro and may contain an alternative mechanism promoting IgE class switching. Alternatively, rapid and abundant expression of IL-4 by basophils has been proposed as a source of IL-4 that may further stimulate TH 2 cell differentiation.
The physiological role of basophils is unknown, although, like other leukocytes, they appear to have a role in host defense. Basophils have long been thought to play a role in tick rejection and are a prominent component of the inflammatory response to many parasites. This proposed role in host defense against the parasite is supported by the recent discovery of functional homologs of the parasite histamine releasing factor in the translationally controlled family of tumor proteins. Although basophils have many characteristics that suggest they contribute to allergic inflammation, the precise role of basophils in the pathogenesis of asthma is unclear. Following allergen challenge, basophils are the predominant IL-4-expressing cell type in human asthmatic airway peripheral blood mononuclear cells.
Eosinophils
Eosinophils are granulocytes that were first described to stain with acid aniline dyes such as eosin. Although these cells are rarely found in the peripheral blood of healthy individuals, eosinophilia in the blood and tissues is a hallmark of helminth infections, allergies, and asthma. Due to the large body of evidence supporting a critical role in the pathogenesis of asthma, the eosinophil has emerged as a major therapeutic target for immunologic therapy of asthma.
Eosinophils typically have a binucleated nucleus with highly condensed chromatin and cytoplasm containing two main types of granules: specific and primary granules. Specific granules have a characteristic ultrastructural appearance consisting of an electron-dense crystalloid core. These granules contain many cationic proteins that give eosinophils their unique staining properties. Primary granules are similar to those found in other granulocyte lineages and are found early in eosinophil development. Eosinophils also contain lipid bodies that play a role in the generation of eicosanoid mediators.
Since there is no cell surface marker specific for eosinophils, their binding to eosin-like dyes remains the most common detection method. The development of monoclonal antibodies to various eosinophil granule proteins has provided an additional means of immunochemical identification of these cells. Eosinophils express a wide range of cell surface molecules, including cytokine receptors (IL-3R, IL-5R and GM-CSFR), chemokine receptors (CCR1 and CCR3), FcγRII (CD32), FcαRI (secretory IgA) complement receptors (C3aR, C5aR, CD88 and CD35), adhesion molecules (very late antigen [VLA]-4 and α4β7 integrin), CD9 and CD69. CD69 is a marker of eosinophil activation and is upregulated on eosinophils isolated from sites of allergic inflammation. Eosinophilic FcϵRI expression is minimal and has unclear functional significance.
Eosinophils develop and mature in the bone marrow from CD34+ progenitor cells and are released into the peripheral blood as mature cells. IL-5, the major eosinophil active cytokine, has profound beneficial effects on the differentiation and proliferation of eosinophil precursors in the bone marrow. Thus, IL-5 is produced peripherally at sites of allergic inflammation, or helminth infection acts distally on the bone marrow. In addition, allergenic stimulation or experimental administration of eotaxin causes the release of mature eosinophils as well as eosinophil precursors in the bone marrow.
After release from the bone marrow, eosinophils circulate in the peripheral blood and then enter tissues with a half-life in the peripheral blood of 8 to 18 hours. Although eosinophils are best known as peripheral blood white blood cells, the vast majority of eosinophils are found in the intestine and lungs. Eosinophils are also the main source of cysteinyl leukotriene LTC 4 and its active metabolites LTD 4 and LTE 4. Eosinophils, along with mast cells and basophils, are the main LTC 4 synthesis-producing cells in the mucous membrane of bronchial asthma. Eosinophils are capable of producing a number of cytokines, including IL-1, transforming growth factor β, IL-3, IL-4, IL-5, IL-8, and TNF-α. However, eosinophils typically produce fewer cytokines than other inflammatory cells such as T cells. As such, the relative contribution of eosinophil cytokine production to the generation of allergic inflammation remains to be determined.
The number of eosinophils in the peripheral blood increases in allergic diseases, asthma and helminthiasis, and tissue eosinophilia is often found at sites of inflammation associated with these diseases. Despite their in vitro activity against parasites, in vivo studies in IL-5 knockout mice generally do not support a significant role for eosinophils in clearing parasitic infections. In allergic diseases and asthma, eosinophils play a proinflammatory role, in which eosinophilic mediators such as MBP are thought to cause mucosal inflammation and consequent bronchial hypersensitivity. Corticosteroids strongly reduce the number of eosinophils in peripheral blood and tissues, further supporting the central role of eosinophils in the pathogenesis of asthma. Because eosinophils are considered the final effector cell of asthma, a number of investigational treatments have targeted eosinophils. Recently, a phase II study of anti-IL-5 in human asthma demonstrated no improvement in either airflow or late-phase allergic reactions, despite a 90% reduction in peripheral blood eosinophils. A subsequent anti-IL-5 study demonstrated a similar 90% reduction in peripheral blood eosinophils, but only a 55% reduction in bronchial mucosal eosinophils. These data demonstrate that anti-IL-5 alone may not be sufficient to reverse pulmonary eosinophilia and that additional anti-eosinophil strategies need to be explored to determine their therapeutic potential in the treatment of asthma.