Metabolic disease
| Lactic acidosis | |
| L - (+) - lactic acid | |
| Speciality | Endocrinology |
Lactic acidosis
a disease characterized by the accumulation of lactate (especially L-lactate) in the body with the formation of excessively low levels in the bloodstream. This is a form of metabolic acidosis in which excessive amounts of acid accumulate due to a problem with oxidative metabolism.
Lactic acidosis is usually the result of an underlying acute or chronic illness, medication, or poisoning. Symptoms are usually related to these underlying causes, but may include: nausea, vomiting, Kussmaul breathing (heavy and deep), and general weakness.
Diagnosis is made on the basis of a biochemical blood test (often initially an arterial blood gas sample), and once confirmed, usually prompts an investigation to determine the underlying cause of the acidosis. In some situations, hemofiltration (blood purification) is required temporarily. In rare chronic forms of lactic acidosis caused by: mitochondrial disease, a specific diet or dichloroacetate may be used. The prognosis of lactic acidosis largely depends on the underlying cause; in some situations (eg severe infections), this indicates an increased risk of death.
Causes
Several different causes of lactic acidosis include:[ citation needed
]
- Genetic conditions Biotinidase deficiency, multiple carboxylase deficiency, or nongenetic biotin deficiencies
- Diabetes and deafness
- Fructose 1,6-bisphosphatase deficiency
- Glucose-6-phosphatase deficiency
- GRACIAL syndrome
- Mitochondrial encephalomyopathy, lactic acidosis and stroke-like attacks
- Pyruvate dehydrogenase deficiency
- Pyruvate carboxylase deficiency
- Leigh syndrome
- Linezolid[4]
- Thiamine deficiency (especially with parenteral nutrition)
Pathophysiology
Glucose metabolism begins with glycolysis, in which the molecule is broken down into pyruvate in ten enzymatic steps. A significant portion of pyruvate is converted to lactate (usually 10:1). The human metabolism produces about 20 mmol/kg lactic acid every 24 hours. This occurs predominantly in tissues (especially muscle) that have high levels of the “A” isoform of the enzyme. lactate dehydrogenase (LDHA), which preferentially converts pyruvate to lactate. Lactate is transported by the bloodstream to other tissues, where it is converted by ATP back to pyruvate by the “B” isoform of LDH (LDHB). First, there is gluconeogenesis in the liver (and also in the kidneys and some other tissues), where pyruvate is converted to glucose; this is known as the Measles Cycle. Additionally, lactate translocated to other tissues enters the citric acid cycle and eventually oxidative phosphorylation, a process that produces ATP.[2]
Increased lactate levels are a consequence of increased production or decreased metabolism. In terms of metabolism, this occurs predominantly in the liver (70%), which explains that lactate levels may be elevated in liver disease.[2]
In “type A” lactic acidosis, lactate production is associated with a lack of oxygen for aerobic metabolism. If oxygen is not available for those parts of glucose metabolism that require oxygen (citric acid cycle and oxidative phosphorylation), excess pyruvate will be converted to excess lactate. In “type B” lactic acidosis, lactate accumulates due to a mismatch between the activity of glycolysis and the remainder of glucose metabolism. Examples are situations where the sympathetic nervous system is very active (such as severe asthma).[2] There is controversy as to whether elevated lactate levels in acute illness can be explained by tissue hypoxia; empirical support for this theoretical view is limited.[12]
Where does lactic acidosis come from?
Lactate is a natural by-product of lactic acid from the breakdown of glucose and amino acids. In a healthy body, it is produced in minimal doses and breaks down into water, carbon dioxide or glucose. Decomposition products are excreted through the kidneys. When their accumulation occurs (lactic acidosis), the acidity of arterial blood increases sharply, the enrichment of cells with oxygen decreases, and the effect of insulin decreases. As a result, hyperglycemic coma occurs.
Based on the nature of the clinical picture and the degree of progression, early, middle and late stages are distinguished. The patient's condition changes in just a few hours, the symptoms change sharply from general malaise to coma. The classification based on etiopathogenetic mechanisms, developed in 1976 by Cohen and Woods, distinguishes several types of lactic acidosis:
- Acquired - type A, manifests itself after 35 years, can develop even in the absence of diabetes. Tissue hypoxia (oxygen starvation of cells) inhibits the functions of the central nervous system and accelerates the respiratory and pulse rates.
- Congenital - type B, occurs due to the underlying disease (diabetes mellitus), toxin breakdown products, and congenital problems with metabolism. Causes kidney or liver failure.
In the international statistical classification (ICD-10), lactic acidosis refers to disorders of water-salt and acid-base balance.
Diagnostics
Acid-base disorders, such as lactic acidosis, are usually first assessed using arterial blood gas analysis. Venous blood testing is also available as an alternative as they are interchangeable.[2] Typically obtained lactate concentrations are in the range given below:[13]
| mg/dl | mm | |
| Deoxygenated blood | 4.5 – 19.8 | 0.5 – 2.2 |
| Arterial blood | 4.5 – 14.4 | 0.5 – 1.6 |
Lactic acidosis is classically defined as elevated lactate levels along with pH [2]
Care
If elevated lactate levels are present in acute illness, maintaining oxygen supply and blood flow are key initial steps.[2] Some vasopressors (drugs that increase blood pressure) are less effective when lactate levels are high, and some agents that stimulate the beta-2 adrenergic receptor may further increase lactate levels.[2]
Direct removal of lactate from the body (eg, with hemofiltration or dialysis) is difficult, with limited evidence of benefit; It may be impossible to keep up with lactate production.[2]
Limited evidence supports the use of sodium bicarbonate solutions to improve pH (which is associated with increased carbon dioxide production and may reduce calcium levels).[2][14]
Lactic acidosis caused by inherited mitochondrial disorders (type B3) can be treated with a ketogenic diet and possibly with dichloroacetate (DCA),[15] although it may be complicated by peripheral neuropathy and has a weak evidence base.[16]
Treatment of lactic acidosis
- Hemodialysis - infusion therapy is used to remove accumulated lactate from the cardiovascular system. Intravenous glucose and insulin injections trigger enzyme and protein activity.
- Artificial pulmonary ventilation (ALV) with intubation of the patient restores gas exchange, maintains impaired respiratory function, and reduces the concentration of carbon dioxide in the blood to normal levels. The procedure is carried out until the alkaline balance is restored.
- Cardiotonic drugs are prescribed to stimulate the activity of the heart muscle and normalize heart rhythm.
- Insulin therapy with prolonged effect.
As an emergency aid, the patient is administered sodium bicarbonate with a concentration of 4% or 2.5%, the daily dose is up to 2 liters. The drug is administered as a slow intravenous infusion, with adequate ventilation and calcium supplementation to mitigate side effects.
For patients with septic shock, crystalloid solutions - saline or saline - are prescribed in an amount of 30 mg/kg for the first 3 hours. Broad-spectrum antibiotics are used to combat concomitant infections.
Metformin
In case of lactic acidosis, the prescription of metformin to patients with type 2 diabetes is associated with the risk of complications due to possible overdose.
If metabolic acidosis is suspected, metformin should be stopped immediately and the patient should be hospitalized. It is important to preserve the vital functions of the body by stabilizing blood circulation and ensuring complete saturation of blood and tissues with oxygen. There is no specific antidote for metformin. In case of overdose, the introduction of activated carbon for adsorption may be indicated after a few hours.
Other animals
Reptiles
Reptiles that primarily rely on anaerobic energy metabolism (glycolysis) during vigorous exercise may be particularly susceptible to lactic acidosis. Particularly during the capture of large crocodiles, the animals' use of glycolytic muscles often causes their blood pH to change to such an extent that they are unable to respond to stimuli or move.[18] Cases have been recorded of particularly large crocodiles that showed extreme resistance to capture later dying due to the resulting pH imbalance.[19]
Some turtle species have been found to be able to tolerate high levels of lactic acid without suffering the effects of lactic acidosis. Painted turtles hibernate, buried in mud or underwater and do not emerge throughout the winter. As a result, they rely on anaerobic respiration to provide most of their energy needs.[20] Adaptations, particularly in the composition of the turtle's blood and shell, allow it to tolerate high levels of lactic acid accumulation. in anoxic conditions, when anaerobic respiration predominates, the level of calcium in the blood plasma increases.[20] This calcium serves as a buffer by reacting with excess lactate to form a precipitate. calcium lactate. It is assumed that this sediment is reabsorbed by the membrane and skeleton, thereby removing it from the bloodstream; in studies of turtles exposed to long-term anoxic conditions, up to 45% of lactate is stored in their skeletal structures.[20]
Ruminants
In ruminant livestock, the cause of clinically severe lactic acidosis is different from the causes described above.
In domestic ruminants, lactic acidosis can result from eating large amounts of grain, especially when the rumen population is poorly adapted to handle grain.[21][22][23] The activity of various rumen organisms leads to the accumulation of various volatile fatty acids (usually mainly acetic, propionic and butyric acids), which partially dissociate.[24] Although some lactate is normally produced in the rumen, it is metabolized by organisms such as Megasphaera elsdenii
and, to a lesser extent,
Selenomonas ruminantium
and some other organisms.
With high grain consumption, the concentration of dissociated organic acids can become quite high, causing the rumen pH to fall below 6. In this lower pH range, Lactobacillus
species (which produce lactate and hydrogen ions) are favored, and
M. elsdenii
and
S. ruminantium
are suppressed , which leads to a significant increase in the concentration of lactate and hydrogen ions in the rumen fluid.[25] The pKa of lactic acid is low, about 3.9, compared, for example, with 4.8 for acetic acid; this contributes to the significant drop in rumen pH that can occur.[24]
Due to the high concentration of solutes in the rumen fluid under such conditions, a significant amount of water moves from the blood into the rumen along the osmotic potential gradient, resulting in dehydration that cannot be corrected by drinking and which can ultimately lead to hypovolemic shock.[21] ] As more lactate accumulates and rumen pH decreases, the concentration of undissociated lactic acid in the rumen increases. Undissociated lactic acid can pass through the rumen wall into the blood,[26] where it dissociates, lowering the pH of the blood. The rumen produces L- and D-isomers of lactic acid;[21] these isomers are metabolized through various metabolic pathways, and the activity of the main enzyme involved in the metabolism of the D-isomer decreases significantly with decreasing pH, resulting in an increase in the ratio of D:L-isomers as acidosis progresses.[25]
Measures to prevent lactic acidosis in ruminants include avoiding excessive amounts of grain in the diet and gradually introducing grain over several days to establish a rumen population that can safely handle relatively high grain intake.[21][22][23] Addition of lasalocid or monensin to feed may reduce the risk of lactic acidosis in ruminants.[27] inhibition of most lactate-producing bacterial species without inhibition of major lactate fermenters.[28] Additionally, using a higher feeding frequency to provide the daily grain ration may allow increased grain intake without reducing the pH of the rumen fluid.[29]
Treatment of lactic acidosis in ruminants may include intravenous administration of dilute sodium bicarbonate, oral administration of magnesium hydroxide, and/or repeated removal of rumen fluid and replacement with water (followed by reinoculation with rumen microorganisms if necessary).[21][22][23]
Symptoms of lactic acidosis
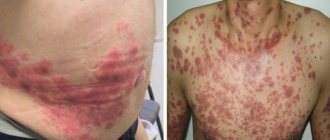
The first signs of pathology are dyspeptic disorders, muscle pain, attacks of sudden pain in the chest, complete ineffectiveness of painkillers. The patient develops disturbances of consciousness. This condition sometimes resembles alcohol intoxication.
With lactic acidosis, abdominal pain often occurs, vomiting, diarrhea, tachypnea and hypothermia are also characteristic. Cardiovascular arrhythmias, hypotension and decreased cardiac output are possible. The neurological picture can be equally varied: from mild confusion, asthenia to deep coma.
If the patient's condition worsens sharply, it is necessary to act immediately:
- Call an ambulance;
- If the patient is breathing and the pulse is palpable, it is necessary to provide him with access to fresh air and place him on his left side;
- If a person is unconscious, the pulse cannot be felt, there is no breathing - proceed to chest compressions;
- If a person is unconscious, do not try to give him something to drink.
Recommendations
- Woods, Hubert Frank; Cohen, Robert (1976). Clinical and biochemical aspects of lactic acidosis
.
Oxford: Blackwell Scientific. ISBN 0-632-09460-5.[ page needed
] - ^ a b c d f f gram h i j k l
Kraut, Jeffrey A.;
Madias, Nikolaos E. (December 11, 2014). "Lactic acidosis." New England Journal of Medicine
.
371
(24):2309–2319. Doi:10.1056/NEJMra1309483. PMID 25494270. - MedlinePlus Encyclopedia
: Lactic acidosis - Santini, A; Ronchi, D; Garbellini, M; Piga, D; Protti, A (July 2017). "Linezolid-induced lactic acidosis: a fine line between bacterial and mitochondrial ribosomes." Expert opinion on the safety of medicines
.
16
(7):833–843. Doi:10.1080/14740338.2017.1335305. PMID 28538105. - Shah, A.D.; Tree, DM; Dargan, P.I. (January 2011). "Understanding Lactic Acidosis in Paracetamol (Acetaminophen) Poisoning." British Journal of Clinical Pharmacology
.
71
(1):20–8. Doi:10.1111/j.1365-2125.2010.03765.x. PMC 3018022. PMID 21143497. - DeFronzo, R. Fleming, Georgia; Chen, K; Bichak, T.A. (February 2016). "Metformin-associated lactic acidosis: current views on causes and risk." Metabolism: Clinical and Experimental
.
65
(2): 20–9. doi:10.1016/j.metabol.2015.10.014. PMID 26773926. - Fimognari, F. L.; Pastorelli, R.; Incalzi, R. A. (2006). "Phenformin-induced lactic acidosis in elderly patients with diabetes: a recurring drama (phenformin and lactic acidosis)." Diabetes care
.
29
(4):950–1. Doi:10.2337/diacare.29.04.06.dc06-0012. PMID 16567854. - "Triumeq (Abacavir, Dolutegravir and Lamivudine Film-Coated Tablets) Drug Information: Description, User Reviews, Drug Side Effects, Interactions - Prescribing Information on RxList." RxList
. Retrieved 2016-03-25. - Truvada.
- McKenzie, Robin; Freed, Michael W.; Sally, Richard; Konjivaram, Hari; Di Bisceglie, Adrian M.; Park, Yoon; Savarese, Barbara; Kleiner, David; Tsokos, Maria; Luciano, Carlos; Pruett, Timothy; Stotka, Jennifer L.; Straus, Stephen E.; Hoefnagle, Jay H. (1995). "Liver failure and lactic acidosis due to fialuridine (FIAU), an investigational nucleoside analogue for the treatment of chronic hepatitis B." New England Journal of Medicine
.
333
(17):1099–105. Doi:10.1056/NEJM199510263331702. PMID 7565947. - Darmon, Michael; Malak, Sandra; Guichard, Isabelle; Schlemmer, Benoit (July–September 2008). "Acute tumor lysis syndrome: a comprehensive review." Revista Brasileira de Terapia Intensiva
.
20
(3): 278–285. Doi:10.1590/S0103-507X2008000300011. ISSN 0103-507X. - Garcia-Alvarez, Mercedes; Marik, Paul; Bellomo, Rinaldo (April 2014). "Stress-hyperlactatemia: current understanding and controversy." Lancet diabetes and endocrinology
.
2
(4): 339–347. Doi:10.1016/S2213-8587(13)70154-2. PMID 24703052. - Goldman, Lee; Schafer, Andrew (11 May 2015). Goldman-Cecil Medicine
(25th ed.). Elsevier. ISBN 978-1455750177. - Boyd, J.H.; Wally, KR (August 2008). "Is there a role for sodium bicarbonate in treating lactic acidosis from shock?" Current Opinion in Critical Care Medicine
.
14
(4): 379–83. Doi:10.1097/MCC.0b013e3283069d5c. PMID 18614899. - Stacpoole, P. W.; Kurtz, T. L.; Khan, Z; Langei, T. (2008). "The role of dichloroacetate in the treatment of genetic mitochondrial diseases". Advanced Reviews of Drug Delivery
.
60
(13–14): 1478–87. doi:10.1016/j.addr.2008.02.014. PMC 3746325. PMID 18647626. - Pfeffer, G; Majamaa, K; Turnbull, D.M.; Thorburn, D; Chinnery, P. F. (2012). Chinnery, Patrick F (ed.). "Treatment of mitochondrial disorders." Cochrane Database of Systematic Reviews
.
4
(4):CD004426. Doi:10.1002/14651858.CD004426.pub3. PMC 7201312. PMID 22513923. - Kajbaf, F; Lalau, J. D. (November 2014). "Mortality rates in so-called 'metformin-associated lactic acidosis': a review of data since the 1960s." Pharmacoepidemiology and drug safety
.
23
(11): 1123–7. Doi:10.1002/pds.3689. PMID 25079826. - Seymour R. S.; Webb G. J. W.; Bennett A. F.; Bradford, D. F. (1987). "Chapter 26. Effects of entrapment on the physiology of Crocodylus porosus
" (PDF).
In Webb, G. J. W.; Manolis, S.C.; Whitehead, P. J. (Ed.). Wildlife Management: Crocodiles and Alligators
. Sydney: Surrey Beatty. pp. 253–257. - [1]. As of January 31, 2009
- ^ a b c
Jackson, Donald S. (2002).
"Hibernation without oxygen: physiological adaptations of the painted turtle." Journal of Physiology
.
543
(3):731–737. doi:10.1113/jphysiol.2002.024729. PMC 2290531. PMID 12231634. - ^ a b c d e
Kimberling, K. W. 1988. Diseases of Jensen and Swift sheep. 3rd ed. Lea & Fibiger, Philadelphia. 394 pp. - ^ a b c
Pugh, D. G. 2002. Medicine for sheep and goats. Saunders. 468 pp. - ^ a b c
Kahn, K. M. (ed.) 2005. Merck Veterinary Manual. 9th ed. Merck & Co., Inc., Whitehouse Station. - ^ a b
Van Soest, P. J. 1994. Nutritional ecology of ruminants. 2nd ed. Cornell Univ. Press, Ithaca. 476 pp. - ^ a b
Nochek J. E. (1997).
"Cattle acidosis: implications for laminitis". J. Dairy Sci
.
80
: 1005–1028. - Owens, FN; Secrist, D.S.; Hill, W.J.; Gill, D.R. (1998). "Cattle acidosis: a review". Journal of Animal Science
.
76
(1):275–86. doi:10.2527 / 1998.761275x. PMID 9464909. - Nagaraja, T.G.; Avery, T.B.; Bartley, E. E.; Galitzer, S. J.; Dayton, A. D. (1981). "Prevention of lactic acidosis in cattle with lasalocid or monensin." Journal of Animal Science
.
53
(1):206–16. doi:10.2527/jas1981.531206x. PMID 7319937. - Dennis, SM; Nagaraja, T.G.; Bartley, E. E. (1981). "Effects of lasalocid or monensin on lactate-producing or utilizing rumen bacteria." Journal of Animal Science
.
52
(2): 418–26. doi:10.2527/jas1981.522418x. PMID 7275867. - Kaufmann W. (1976). "The influence of diet composition and feeding frequency on ruminal pH regulation and feed intake in ruminants." Livestock Prod.
The science .
3
: 103–114.