Multiple myeloma is a type of blood cancer, although the main manifestations of this disease are associated with bone damage. The basis of the disease is damage to a certain type of leukocyte (plasmocyte). Like all other blood cells, plasma cells are formed from stem cells in the bone marrow. The formation process is a series of successive divisions in which lymphoid stem cells are formed first, and then B lymphocytes.
B lymphocytes are immune cells that fight bacteria and viruses that are foreign to the body. These cells finally mature outside the bone marrow: in the thymus, spleen and lymph nodes. To do this, they need an antigen, which is a protein of a foreign microorganism. Contacting such an antigen, the B lymphocyte becomes a plasma cell, beginning to secrete antibodies - special proteins that can destroy foreign cells. Each plasma cell is capable of secreting a specific type of antibody aimed at fighting a specific microorganism. During normal functioning of the body, a person produces a certain and strictly controlled number of plasma cells.
General information
Multiple myeloma or myeloma, what is this disease?
This is an oncohematological disease that is characterized by the uncontrolled proliferation of clonal plasma cells. Plasma cells are concentrated in the bone marrow, bones (a destructive process occurs) and internal organs (their function is impaired). The damage to the skeletal bones is extensive and is localized in the skull, pelvic bones, ribs, and spine. Bone destruction is accompanied not only by pain, but also by spontaneous fractures. A plasma cell is a differentiated B lymphocyte that is involved in the production of antibodies in the formation of immunity .
Tumor plasma cells synthesize monoclonal immunoglobulin (it is called M-protein or M-complex, found in blood and urine). Its structure is not damaged, but its synthesis exceeds physiological norms. Multiple myeloma is considered a disease of older age. The maximum occurrence occurs between 50-70 years. However, it can also occur in young people. Patients under 40 years of age account for 5-10%. Men get sick more often. Synonyms for this disease are myelomatosis and plasma cell myeloma - the term “multiple myeloma” has been changed to this term. Multiple myeloma is coded in ICD-10 as C90.0.
Paradigm Shift
Every year in Russia, myeloma is diagnosed in 4.5 thousand people (counting only new cases of the disease). For most patients, encountering a diagnosis comes as a huge shock, because it soon becomes clear that this is an incurable disease. And that even despite the most modern and timely treatment, sooner or later you will inevitably have to face a relapse of the disease. Therefore, according to psychologists, 30-35% of people with this diagnosis suffer from depression and anxiety disorders. Over time, this number becomes even higher, since the drugs used to treat MM themselves can increase the risk of depression. Therefore, in addition to oncohematologists, psychotherapists often take part in treatment.
Which pills increase the risk of cancer? More details
However, today an unprecedented breakthrough has occurred in oncohematology. And in particular, people with multiple myeloma, who until recently were doomed to quick death (even with the disease detected at an early stage, patients lived only a few years), today, thanks to innovative biologically targeted drug therapy, they can live quite a long and high-quality life. Just 7 years ago, the 5-year disease-free survival rate for MM was 36%, and today it reaches 50%.
Pathogenesis
The development of plasma cell myeloma occurs at the level of early B-lymphocyte precursors and the primary transformed cells differentiate to plasma cells (final stage). As they mature, they begin to secrete immunoglobulins. Tumor cells have their own growth patterns. In the early stages, tumor growth doubles in 2-3 days. During the period of its clinical detection, the mass is 1 kg/m2, while the number of rapidly growing cells decreases. Tumor growth sometimes stops with a smaller mass, then a quiet and long-lasting course is observed. Signaling systems function in myeloma cells and surrounding tissue.
M protein, which is produced by plasma cells, most often refers to IgG, as well as IgA. But regardless of their type, 40% of patients develop Bence-Jones proteinuria (monoclonal light chains are detected in the urine). In 20% of patients, malignant cells secrete only this B-D protein, and in the non-secretory form, M-protein is not detected in the urine and blood at all. The disease is characterized by the development of osteoporosis, most often in the pelvic bones, vertebrae and ribs. This is due to the fact that normal bone is replaced by a growing tumor, and cytokines secreted by malignant cells activate osteoclasts, which destroy bone. Increased bone loss is accompanied by increased calcium in the blood.
Extraosseous plasmacytomas may occur in the upper respiratory tract. Many patients already at the time of diagnosis have renal failure , which develops due to the deposition of light chains in the kidney tubules, as well as increased levels of calcium in the blood. Anemia is associated with kidney disease and suppression of erythropoiesis .
Recovery process
Any of the treatment regimens involves the use of drugs with a large number of side effects. The body weakened by the disease also reacts in a certain way to autotransplantation: neutropenia or thrombocytopenia may be observed in the blood for a long time. In addition, several weeks after autotransplantation, the development of infectious complications and exacerbation of chronic diseases may occur. Manifestations of asthenic syndrome are observed.
Thus, the recovery process after treatment may take some time. The presence and severity of symptoms during recovery should be taken into account when choosing further therapy, as well as when deciding on repeat autotransplantation.
Classification
Plasma cell tumors include:
- Multiple myeloma.
- Plasma cell myeloma and its variants (asymptomatic, non-secreting plasma cell leukemia).
- Plasmacytoma.
According to the activity of the disease, there are:
- “Smoldering” (asymptomatic). This form does not manifest symptoms characteristic of the disease (there are no foci of bone destruction and damage to internal organs). The M component is more than 30 g/l or the number of plasma cells in the bone marrow is more than 10%. The risk of progression to symptomatic disease is high.
- Active myeloma. There is active tumor growth, osteodestruction , increased blood viscosity, and infectious complications appear.
According to the clinical and anatomical classification, multiple myeloma comes in several forms:
- Diffuse.
- Multifocal.
- Diffuse-focal form.
- Mainly visceral.
Multiple myeloma (ICD-10 code C90.0) has stages that differ in the degree of production of pathological protein:
- Stage I. Low production of M-protein (IgG less than 30 g/l), tumor weight up to 600 g/m2, protein B-D less than 4 g/s, normal bone structure or there is one lesion.
- Stage II. The tumor weight is 600-1200 g/m2 and the rate of M-complex production is average.
- Stage III. Low hemoglobin level, elevated calcium, multiple bone lesions, IgG more than 70 g/l, and protein B-D above 12 g/day.
The blood disease myeloma can be quiet and symptomatic with bone damage, bone marrow suppression and kidney failure. Symptomatic myeloma blood disease is characterized by high production of paraprotein and it corresponds to the “detailed” picture of the disease and necessarily requires treatment. Impaired renal function is always present and, depending on the level of creatinine, all patients are divided into three groups to determine the prognosis.
Main criteria of the disease:
- Detection of more than 10% plasma cells in the bone marrow or the development of a plasma cell tumor.
- The presence of monoclonal immunoglobulin , which is produced by malignant cells.
- It is determined by electrophoresis of blood proteins. In practice, they focus on a value of 30 g/l IgG. it is necessary to constantly monitor the level of M-protein, since its increase is a progression factor.
- Often, patients monoclonal immunoglobulin , but there is a decrease in the level of gammaglobulin in the blood and when examining urine, the release of light k-chains is noted.
When organs and tissues are damaged, the following develop in parallel:
- Calcium levels increase.
- Renal function is impaired (the main criterion is increased creatinine).
- Anemia develops .
- Bone damage (compression of vertebral bodies, lysis, fractures).
Damage in myeloma is diffuse and focal. In the diffuse form, only the bone marrow is affected, and this form is detected in 24% of cases. The diffuse-focal form of multiple myeloma is a mixed variant and combines the presence of tumor growth in the body and damage to the bone marrow. This option is most common.
Nonsecretory myeloma is said to occur when plasma cells are detected in the bone marrow and there are signs of organ damage. But with this form there is no monoclonal protein in the blood and urine, so kidney failure rarely develops.
Asymptomatic or smoldering form. It is characterized by the following criteria: M protein more than 30 g/l, 500 mg of paraprotein in the urine or more than 10% of plasma cells in the bone marrow. There is no damage to organs and bones, and there is no amyloidosis . In this case, treatment is not carried out, but patients are observed once a quarter (paraprotein in the blood and urine is determined). Typically, the interval before disease progression is 4 years.
Plasmacytomas are plasma cell tumors. Depending on the location, they can be bone (bone plasmacytoma) or extramedullary (soft tissue is affected). Solitary plasmacytoma is represented by a single focus in the bone or tissue (it happens that the production of M protein is low). The diagnosis of bone plasmacytoma is made if the tumor is located in the bone and there are no other systemic signs. The disease is more common in young people. Solitary lesions are located in the vertebrae ( vertebral plasmacytoma ) and tubular bones. Patients are concerned about pain in damaged bones and pathological fractures. For solitary plasmacytoma, radiotherapy is used. In 50%, transformation to multiple myeloma is noted within 3 years.
Cutaneous plasmacytoma or extramedullary myeloma of the skin has a favorable course. Skin lesions in extramedullary myeloma occur in 4% of patients. Unlike multiple myeloma, cutaneous plasmacytoma occurs without damage to the bone marrow and organs. If there is no hypercalcemia and no bone marrow involvement, their life expectancy can reach 10 years. But there are observations when, with cutaneous myeloma, myelomatosis develops after a few years . There are also opposite cases - myeloma metastasizes to the skin from the bone marrow. This fact is considered as an unfavorable sign, and the life expectancy of patients can be 1.5-12 months.
How is multiple myeloma treated?
Multiple myeloma is now considered an incurable disease. Medical efforts are aimed at containing tumor growth, prolonging and improving the quality of life of such patients.
After the diagnosis has been established, it is necessary to decide whether the patient needs specific treatment, or whether observation can be limited, since with “smoldering myeloma” (there are no symptoms, but there are laboratory changes), a wait-and-see approach is possible.
The following types of treatment for myeloma are distinguished:
- Standard chemotherapy. Prescribed to patients for whom high-dose chemotherapy is contraindicated. The average life expectancy after it is 29 months. The standard first-line regimen includes melphalan and prednisolone. More effective regimens include thalidomide, lenalidomide, or bortezomib (relatively new anticancer drugs).
- High-dose polychemotherapy (HDPT) followed by transplantation of hematopoietic stem cells HSCT (both autologous and donor). This treatment allows to achieve complete remission in most patients (up to 75%), but unfortunately, progression of the disease is observed within 2-5 years. It is more effective to conduct a double course of HPCT with HSCT (tandem HPCT). It allows achieving five-year disease-free survival in 90% of patients. But not all patients can tolerate such severe treatment, so indications for it are limited.
- Maintenance therapy. Even VHD cannot prevent the development of relapse; treatment is prescribed, which is designed to suppress the clone of malignant cells. Interferons are used for this purpose. They help extend the median disease-free survival to 42 months.
- Combating complications. Pain treatment is the prescription of strong analgesic drugs, radiation therapy. Surgical operations are performed for compression fractures of the vertebrae. Correction of complications caused by inhibition of hematopoiesis - transfusion of red blood cells and administration of erythropoietin, use of antibiotics for fever. Carrying out hemodialysis, plasmapheresis, prescribing bisphosphonates to control hypercalcemia.
Causes
The occurrence of multiple myeloma is a complex process that involves a combination of external and internal factors. Permissive factors for the development of the disease are:
- Long-term antigenic stimulation that develops after viral infections and chronic diseases.
- Exposure to radiation and toxic substances.
- Infection with type 8 herpes virus .
- If we consider the genetic factor, then with genetic changes a clone of plasma cells is formed, which produces immunoglobulin . A sequence of genetic events in clonal cells causes their uncontrolled division and progression of the nascent tumor. Chromosomal translocations are the main factor that activates proto-oncogenes . In multiple myeloma, complex combinations of chromosome changes (in number and structure) are detected. Clinical manifestations of the disease are associated with genetic changes, and the prognosis is influenced by mutations of the K-ras and N-as oncogenes. When talking with the patient, attention is paid to the family history - usually their medical history includes relatives who have hematological diseases - lymphomas and chronic lymphocytic leukemia .
Causes and risk groups
The exact causes leading to the occurrence of this disease have not been identified. Presumable etiological factors are:
- Ionizing radiation.
- Prolonged contact with toxic substances.
- Long-term antigenic stimulation.
- Infection with herpes virus type 8.
The effect of a hereditary factor for myeloma is doubtful, but sometimes it occurs in members of the same family.
Risk factors for multiple myeloma include:
- Age over 40 years (average age of patients is 65 years).
- Male gender.
Symptoms of multiple myeloma
The duration of the disease before the first symptoms appear can be from months to 3 years. Multiple myeloma differs from other blood diseases in that the symptoms of multiple myeloma are varied, since the tumor itself and its products cause numerous changes in various organs. In 20% of patients, there are no signs of myeloma (asymptomatic), but an increased ESR appears. Then bone pain, weakness and weight loss appear. Bone pain is the most common symptom and occurs in 70% of patients and appears very early.
Often the disease begins suddenly with the appearance of pain in the skeleton or a fracture. Foci of bone changes on an x-ray are defined as lysis (bone destruction). Bone destruction is associated with the proliferation of the tumor clone, in addition, osteoclasts are activated. This process primarily develops in flat bones (the skull, sternum, ribs, pelvic bones) and the spine. Destruction of the vertebrae is manifested by radicular syndrome in different parts of the spine.
Pain bothers the patient when moving, and metastases of various tumors to the bone intensify at night. Constant local pain indicates fractures. Bone lysis and proliferation of the tumor mass are so pronounced that tumors are palpable on the skull or sternum. Bone lysis is accompanied by a loss of calcium from bone tissue and an increase in calcium and phosphorus in the blood. This fact explains the appearance of drowsiness , nausea and vomiting .
Subsidence of the vertebrae is accompanied by compression of the spinal cord (“collapse of the spine”), and a lesion in the cauda equina region disrupts the functioning of the pelvic organs. Compression of the spinal cord is accompanied by loss of sensation, weakness of the muscles of the arms and legs, paresis and plegia . Neuropathy (burning, tingling in the arms and legs, loss of sensitivity) is associated with the impregnation of the nerves with amyloid.
Kidney pathology is observed in 50% of patients and is associated with the filtration of pathological protein (the so-called light chains) in the glomeruli, which is excreted in the urine. A large number of them damage the tubules and lead to kidney failure , which shortens life expectancy. Blood creatinine greater than 173 mmol/l is diagnostically significant. There is also infiltration of the kidneys by plasma cells, so the kidneys increase in size. High calcium levels lead to mental disorders and coma . Hypercalcemia is considered one of the causes of renal failure. A common symptom is a tendency to bacterial infections. Increased blood viscosity is accompanied by headache , blurred vision and changes in the fundus.
The development of anemia is explained by the replacement of hematopoiesis by plasma cells. The presence of anemia sometimes becomes a reason to search for its cause, and after conducting other studies, the correct diagnosis is established.
The diagnosis is established by an increase in total protein, protein in the urine, anemia, or the presence of renal failure .
Tests and diagnosis of myeloma
- General urine analysis. It only detects the presence of protein in the urine.
- Electrophoresis and immunophoresis of urine, which allows you to detect light chains and quantify them.
- To perform the analysis, urine is collected for 24 hours. In 20% of patients, only light chains are detected in the urine (and blood) - a diagnosis of “light chain myeloma” is made.
- Clinical blood test. In the initial stage, there may be no changes in the blood, but as the process generalizes, normochromic anemia develops, sometimes being the main manifestation of the disease.
- A characteristic symptom is a sharp increase in ESR to 60-90 mm/h.
- Biochemical blood test (calcium, creatinine, urea, uric acid, sodium, potassium, total protein and fractions).
- Determination of the level of monoclonal immunoglobulin (paraprotein). This is a complete immunoglobulin , which consists of light and heavy chains. In myeloma, increased secretion of immunoglobulin IgG is more often detected.
- The level of free light chains of immunoglobulins in the blood (FLC kappa and lambda), which increases in multiple myeloma. Tumor cells in myeloma secrete light chains of immunoglobulins, so their complete assembly does not occur. The free light chains are called Bence Jones protein . Multiple myeloma that produces only light chains is called Bence Jones myeloma.
- Determination of light chains of immunoglobulins in urine (Bns-Jones protein).
- Diagnosis necessarily includes bone marrow puncture - one of the leading methods. The abundance of plasma cells is determined in the bone marrow punctate. The diagnosis is reliable when more than 10% of clonal plasma cells are detected.
- Cytogenetic study to detect chromosomal changes.
- X-ray of bones (spine, skull, pelvis, humerus and femur).
- MRI of the spine or pelvis is performed in patients with suspected solitary plasmacytoma or spinal cord compression.
- to assess the severity of osteoporosis .
Discussion
An analysis of published studies assessing the effectiveness of treatment for MM shows a clear correlation between achieving a deep antitumor response and progression-free survival [1–11]. The results of our study also demonstrate the important role of achieving MRD-negative status after auto-HSCT, which is associated with the best RFS.
An open issue in modern treatment tactics for patients with MM remains the need for maintenance therapy for patients who have achieved CR, including after auto-HSCT. Currently, the results of foreign studies are available on the analysis of MRD in patients with MM after auto-HSCT [19]. There are very few similar studies among domestic publications [23, 24]. So, Yu.S. Osipov et al. [24] studied the presence of MRD in patients with MM who achieved CR after auto-HSCT. CM of 20 patients was studied using 3-color flow cytometry. As a result, it was determined that the three-year DFS in the group of MRD-negative patients was 68% versus 0% in the group of MRD-positive patients. The authors came to the conclusion that it is necessary to prescribe maintenance therapy to patients with MRD-positive status after auto-HSCT due to the high risk of early relapse [24].
The results of our study made it possible to determine clear indications for maintenance treatment of patients with CR after auto-HSCT - the presence of MRD on the 100th day after transplantation. Maintenance therapy with bortezomib ensured the achievement of MRD-negative status in another 42% of patients, in whom 0.01% of aberrant cells were detected 100 days after auto-HSCT. In addition, during maintenance therapy, the average time to disease relapse increased: 17.3 ± 1.8 months versus 12.3 ± 1.8 months.
In patients who achieved MRD-negative status after auto-HSCT, maintenance therapy with bortezomib did not have a significant positive effect. The two-year ESR in the group of MRD-negative patients receiving maintenance therapy was higher than in the group of patients without maintenance treatment. This may be due to the small number of patients in the compared groups and the small number of censored outcomes. It should be noted that we intentionally carried out randomization in a 1:2 ratio, since generally accepted foreign clinical practice and most clinical studies leave no alternative to the issue of providing maintenance treatment to all patients with MM after auto-HSCT.
The results of our pilot study showed that without maintenance treatment in the group of MRD-negative patients there were no early relapses of the disease. Therefore, we do not see the absolute advisability of prescribing maintenance therapy with bortezomib to patients who have achieved a strict CR or MRD-negative status after auto-HSCT.
In children
Cases of multiple myeloma in children are considered rare, since it is 140 times less common in childhood. Diagnosis of this disease in children is difficult due to its rare occurrence at this age and the reduced alertness of doctors. The diagnosis is made by detecting myeloma cells in the bone marrow and a typical x-ray picture. The clinical picture does not differ from that in adults, but in children the lymph nodes are more often involved, which is associated with the generalization of the process. The course of the disease in children is steadily progressive. Average life expectancy is 2-3 years.
To prevent myeloma from breaking
Oncohematologists and psychologists advise patients to treat their disease not as incurable, but as chronic. Myeloma requires constant monitoring and treatment, but you can live with this disease. In principle, in this regard, this formidable pathology is not much different from hypertension, coronary heart disease, diabetes mellitus, rheumatological diseases and other chronic diseases characterized by constant periods of exacerbation and remission. And yet, there is no need to put your illness in first place, where by right every person should have his own life with its interests and joys, goals and achievements. This attitude will definitely help you cope with any difficulties!
Diet
Diet 15 table
- Efficacy: therapeutic effect after 2 weeks
- Timing: constantly
- Cost of food: 1600-1800 rubles per week
Nutrition for this disease must be complete in essential nutrients and have sufficient energy value. Complete protein in sufficient quantities is important in the diet (beef, poultry, rabbit, turkey, liver, fish, cottage cheese). The patient should receive 2 grams of protein per kilogram of weight daily.
Steamed meatballs, meatballs or cutlets are prepared from meat products. Sources of calcium include fermented milk products, cottage cheese, sunflower and sesame seeds. The daily diet should include foods containing vitamins B and C, and, taking into account anemia, foods rich in iron (beef, beef liver, pomegranate). Meals should be fractional - small portions for better digestion and to avoid digestive overload.
Simple carbohydrates are excluded, since taking dexamethasone and prednisolone may increase sugar levels. You should not eat fatty, fried and spicy foods, fatty milk and cream, rich soups, smoked foods, canned food and pickles. During the period of remission, nutrition can be more varied and strict restrictions are not provided. Vitamins and microelements are still important in the diet, the sources of which are green vegetables, fruits, flaxseed and olive oil, fish oil, and a variety of nuts.
Consequences and complications
The consequences and complications of this disease include the following:
- Anemia.
- Hypercalcemia.
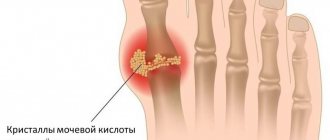
- Increased uric acid levels.
- Bacterial or viral infections that are very severe due to immunodeficiency.
- Kidney failure.
- Lytic bone lesions and osteoporosis . In most cases, patients are indicated for intravenous administration of bisphosphonates (zoledronic acid). They reduce bone pain and various complications associated with osteoprosis. In case of reactions to the administration of biosphosphonates, denosumab Prolia drug monthly, subcutaneously) can be used.
- Spontaneous bone fractures.
- Spinal cord compression. It is the result of tumor growth from the vertebrae. Patients experience pain as with radiculitis , loss of sensitivity, paresis of the legs and even dysfunction of the pelvic organs. Due to compression fractures, there is a risk of pulmonary infections.
- Multiple thromboses due to increased blood viscosity.
- Disintegration of a large tumor.
- In the terminal stage - paraproteinemic coma .
Prognosis for blood myeloma: can it be cured?
The problem of “how to recover completely” remains unresolved for patients. This is a potentially fatal disease because without treatment the life expectancy is no more than 6 months. With standard chemotherapy, survival increased to 3 years, but complete and long-term remissions in multiple myeloma were rare. With the advent of immunomodulators ( Revmilid ) and protease inhibitors ( Velcade ), life expectancy has increased
The use of Velcade has changed the prognosis of this disease. In the last decade, life expectancy with myeloma has increased in stage I to 6-7 years (in some cases more than 15 years), and in stage II up to 4 years. The same cannot be said about stage 3 plasmacytoma. Life expectancy for grade 3 multiple myeloma is the lowest - no more than 2 years. This is due to the fact that this is a stage of pronounced changes in the body: low hemoglobin , high levels of calcium and paraprotein , pronounced bone destruction, and the tumor mass itself is large.
The prognosis for multiple myeloma depends on many factors. Unfavorable factors that are detected already at the time of diagnosis of the disease include low albumin , elevated lactate dehydrogenase , elevated beta-2-microglobulin and cytogenetic abnormalities . The prognosis for patients with renal failure is also unfavorable.
Stages of myeloma
Immediately after identifying the disease, doctors determine its stage - they find out how seriously the body has suffered. They need this information to provide approximate prognoses for the patient and select the most suitable treatment for him.
Myeloma staging is based on 4 factors:
- albumin
in the blood is a protein that maintains pressure, delivers various chemical compounds to tissues and is involved in metabolism. - b2-microglobulin
in the blood is a protein present on the surface of almost all cells of the body. Its content in the blood increases during inflammatory processes, myeloma, lymphoma and some other diseases. - The amount in the blood of LDH, lactate dehydrogenase
, a protein found in almost all cells of the body that is involved in the production of energy from glucose. - Cytogenetic abnormalities
in altered cells: incorrect number or disruption of the structure of chromosomes, each of which represents one DNA molecule storing genetic material. Such changes may mean a worsening prognosis. For example, the loss of part of chromosome 17, as well as the exchange of material between 4 and 14, or between 14 and 16, are considered high-risk because they significantly worsen the patient's prospects. All other anomalies are classified as standard risk.
In total, doctors distinguish 3 stages of myeloma
:
I:
Albumin
level 3.5 grams/deciliter 1 deciliter = 100 cubic centimeters, or 1/10 of a liter or higher,
b2-microglobulin
less than 3.5 mg/l,
normal LDH
II:
The second stage of the disease includes all conditions whose indicators cannot be attributed to stages I or III.
III
B2-microglobulin
level is 5.5 mg/L or more, high-risk cytogenetic abnormalities, and/or high
LDH
.
List of sources
- Mendeleeva L.P., Votyakova O.M., Rekhtina I.G. Multiple myeloma. Russian clinical guidelines for the diagnosis and treatment of malignant lymphoproliferative diseases. Ed. I.V. Poddubnoy, V. G. Savchenko. M., 2021. P.: 213–41.
- Bessmeltsev S.S. Multiple myeloma (pathogenesis, clinical picture, diagnosis, differential diagnosis) // Clinical oncohematology. — 2013.— T6, No. 3.— P. 237-257.
- Bessmeltsev S.S., Abdukadyrov K.M. Multiple myeloma. A modern view of the problem. Almaty, 2007.
- Moiseev S.I., Salogub G.N., Stepanova N.V. Modern principles of diagnosis and treatment of multiple myeloma. – St. Petersburg: St. Petersburg State Medical University Publishing House, 2006. – 39 p.
- Votyakova O.M. Multiple myeloma: achievements of drug treatment of the XXXXI centuries // Oncohematology. – 2004. – T. 6, No. 4 – P. 1925.
Myeloma treatment
The fight against myeloma is a complex task that requires the participation of many doctors of various specialties: oncologist, chemotherapy, neurologist, urologist and others. The more information about the state of the body and the course of the disease they receive from each other, the more factors they are able to take into account.
The oncology department provides complete diagnosis and any treatment for myeloma. We consider each case individually - all our patients receive therapy prescribed by the decision of a council of doctors, candidates and doctors of medical sciences with extensive experience. This approach allows us to select the best options for influencing altered cells and causing minimal harm to healthy tissues. We use only the most modern equipment and original drugs that give predictable results. With us you don’t have to waste time - examination and treatment are carried out with us without queues or delays, just on time.
Doctors use several methods to treat myeloma:
Drug therapy
Chemotherapy
is the destruction or prevention of the growth of altered cells using special drugs.
These include: Cyclophosphamide, Etoposide, Doxorubicin, Melphalan and Bendamustine. They enter the bloodstream and reach almost all areas of the case, affecting any foci of the disease. Corticosteroids
, powerful anti-inflammatory drugs such as Dexamethasone and Prednisolone, are an important part of therapy.
They can be used alone or in combination with other medications. Immunomodulators
are substances that affect a person’s own immune system and help it fight the disease: Lenalidomide and Pomalidomide.
Proteasome inhibitors
- prevent the breakdown of proteins necessary for cell division: Bortezomib, Carfilzomib and Ixazomib.
Histone deacetylase inhibitors
, such as Panobinostat, affect the activity of certain genes in cells.
Monoclonal antibodies
:
substances artificially designed to attack a specific target—for example, proteins on the surface of myeloma cells. These include Daratumumab and Elotuzumab. Bisphosphonates
are medications that slow down the weakening of bones and reduce pain in them: Pamidronate. Zoledronic acid and Denosumab.
Stem cell transplantation
– a bone marrow transplant, whose cells can become red blood cells that carry oxygen throughout the body, white blood cells that help fight infections, and platelets that stop bleeding. First, the patient is given high-dose chemotherapy, which destroys the damaged bone marrow, after which new, healthy hematopoietic cells are injected. Transplantation can be autologous - using pre-collected own material, or allogeneic - using cells from another person - a donor.
Radiation therapy
: destruction of foci of disease using radiation is one of the most suitable options for combating single plasmacytomas. Plasmacytoma is a tumor consisting of altered plasmacytes - cells that produce antibodies - proteins that protect the body from bacteria and viruses.. It is used to treat areas of bones damaged by myeloma that have not responded to chemotherapy or other drugs are painful or may break down.
IVIG
maintenance therapy is the intravenous administration of normal antibodies - immunoglobulin proteins necessary to fight infection.
Erythropoietin
is a drug that increases the number of red blood cells that carry oxygen from the lungs to other tissues.
Plasmapheresis
is a procedure that removes myeloma proteins and reduces blood viscosity.
7th course of chemotherapy for a rare disease - myeloma.