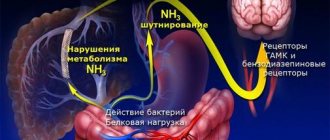
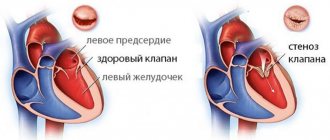
What is Restless Legs Syndrome?
Restless legs syndrome is a pathological condition consisting of an obsessive desire to move the legs, caused by a number of certain unpleasant sensations in the lower extremities. These sensations with restless legs syndrome can be pain or aching in the muscles, as well as itching, a burning sensation, crawling distension or twitching in the lower extremities.
Restless legs syndrome
These subjective symptoms more often occur at night or when taking a horizontal body position and relaxing. A significant weakening of the above symptoms occurs with contraction of the muscles of the lower extremities. Therefore, patients with restless legs syndrome have an obsessive need to move the lower extremities.
Causes
In most cases, RLS occurs in the absence of any other neurological or physical disease. RLS can be primary (ideopathic) or secondary (associated with various pathological conditions).
Medical conditions that may cause secondary RLS.
- Pregnancy
- Peripheral neuropathy
- Iron deficiency
- Radiculopathy
- Kidney failure
- Parkinson's disease
- Spinal cord injuries
- Diabetes
- Postgastrectomy syndrome
- Rheumatoid arthritis
- Abuse of strong drinks, coffee, tea and smoking.
It should be noted that not all patients with these conditions experience RLS. Sometimes there are familial cases when the disease is observed in several generations.
Treatment for restless legs syndrome
Taking multivitamins, anticonvulsants, and giving up bad habits often help get rid of restless leg syndrome.
Restless legs syndrome is a fairly common complaint in patients suffering from varicose veins for a long time. Chronic venous insufficiency, which occurs with varicose veins, disrupts the electrolyte balance in the tissues of the lower extremities, leading to the appearance of restless legs syndrome. This condition is well known to many patients with varicose veins. In some cases, wearing compression stockings may help, providing some relief. However, after some time, restless legs syndrome returns. Only radical treatment of varicose veins helps patients completely get rid of restless legs syndrome.
Restless legs syndrome is not a symptom exclusively of varicose veins. There are many other causes of restless legs syndrome. It is very likely that a therapist or neurologist will be able to help. However, if you have restless legs syndrome, it is better to visit a phlebologist and undergo a diagnosis of the venous system of the lower extremities.
Diagnosis of restless legs syndrome
If you have varicose veins, lifestyle changes and vitamin supplements will not be able to completely rid you of restless legs syndrome. Only good treatment for varicose veins will be an effective remedy for restless legs syndrome in this situation.
Hope for the future
Although there is no targeted treatment for this condition, researchers are working to learn more about RLS.
“Even though scientists have discovered these facts, treating RLS remains challenging. Since iron deficiency occurs in the brain, it is not easy to get it there. We are looking at iron treatments, trying oral and intravenous iron. Sometimes treatment doesn't work. There is an assumption that iron deficiency can change genetics or other processes in the body, so even after replenishing the deficiency, the patient's condition remains the same. We are working on new iron therapy treatments for people who do not respond to standard therapy. In addition, other treatments for RLS are being explored.”
Patient Questions About Restless Legs Syndrome
My legs twitch when falling asleep, what should I do?
If your legs twitch when falling asleep, you need to figure out the cause of this phenomenon. Convulsive contractions of the muscles of the lower extremities can be caused by a number of reasons. First you need to visit the following specialists: a neurologist and phlebologist. An ultrasound duplex examination of the veins of the lower extremities must be performed. Having understood the cause of the condition, it will be possible to completely get rid of convulsive twitching at night.
How to treat restless legs syndrome?
To understand how to treat restless legs syndrome, it is necessary to find out the cause of its occurrence. This will require consultations with the following specialists: a neurologist, phlebologist and therapist.
My legs twist a lot at night, what should I do?
If you experience severe leg cramps at night, seek medical attention. A very common cause of this condition is chronic venous insufficiency, complicating varicose veins.
Pain in legs at night when lying down, which doctor should I go to?
If you have pain in your legs at night when lying down, the first doctor you should visit is a phlebologist. At the beginning, it is necessary to exclude venous pathology. Perhaps a phlebologist will completely solve your problem.
Idiopathic hypereosinophilic syndrome
In recent years, in the clinical practice of doctors of various specialties, diseases and syndromes accompanied by high eosinophilia in peripheral blood have become increasingly common. In this case, special attention is drawn to the so-called large blood eosinophilia, in which the number of eosinophils exceeds 1500 in 1 μl [1, 2, 3]. Long-term eosinophilia can lead to a pathological condition that acquires the features of a separate nosology. The authors describe the etiology, pathogenesis, clinical picture, diagnosis and principles of treatment of idiopathic hypereosinophilic syndrome (IHES) and reactive eosinophilia. From a practical point of view, the diagnosis and treatment of IHES, a serious disease that requires long-term therapy, pose particular difficulties.
Rice. 1. Etiological classification of eosinophilia
With eosinophilia, there is a shortening of cell cycles in the early stages of eosinophil maturation, the mitotic index increases, the generation time of eosinophilic leukocytes is reduced by 3 times, and the time of appearance in the bloodstream is 2 times. In addition, eosinophils are able to return to the bloodstream from tissues and recirculate for a long time (T1/2 - 44 hours) [4].
Functions of eosinophils
The eosinophil as a separate cellular element was first described by Paul Ehrlich in 1879. It was he who used the acidic dye eosin, named after the Greek goddess of the dawn, for histological staining of blood and tissues. P. Ehrlich showed that eosinophils account for 1 to 3% of peripheral blood leukocytes in healthy individuals.
Over the next 40 years, much information has accumulated about eosinophils: increased numbers of cells have been associated with bronchial asthma and helminth infestation. In addition, it was found that the number of eosinophils in animal tissues increased significantly after an anaphylactic reaction. This suggested that eosinophils mediate hypersensitivity during anaphylaxis. This hypothesis remained the main explanation for eosinophil function from the early 20th century until the 1980s.
According to modern concepts, eosinophils are non-dividing granulocytes, which, like other polymorphonuclear leukocytes (PMNL), are continuously formed in the bone marrow from a single stem cell. Eosinophilopoiesis and the differentiation of eosinophils from progenitor cells are regulated by T lymphocytes through the secretion of granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-3 (IL-3), and interleukin-5 (IL-5). In addition, IL-5 and GM-CSF activate eosinophils, inducing a transition of cells from normal to low density (less than 1.085).
The lifespan of eosinophils is 10–12 days. After leaving the bone marrow, where they are formed and mature within 3–4 days, eosinophils circulate in the blood for several hours (their half-life is 6–12 hours). Then, like neutrophils, they leave the bloodstream and enter perivascular tissues, mainly the lungs, gastrointestinal tract (GIT) and skin, where they remain for 10–14 days. For every peripheral blood eosinophil, there are approximately 200–300 eosinophils in the bone marrow and 100–200 in other tissues.
According to light optical examination, the diameter of eosinophils is 12–17 μm; they are usually somewhat larger than neutrophils. Unlike mature polymorphonuclear leukocytes, whose nuclei have about four lobules, the nuclei of eosinophils, as a rule, consist of two lobules connected by a thread. The main peculiarity of their cytoplasm is the presence of two types of specific granules (large and small), which are red or orange. Even in poorly stained smears, eosinophils can be distinguished from neutrophil granules because they are more numerous and distinctly larger. The large granules contain major proteins unique to eosinophils: large basic protein (LBP), eosinophil cationic protein (ECP), eosinophil peroxidase (EPO), eosinophil neurotoxin (EN), formerly called eosinophil protein X, and BOP homologue. The small granules contain the enzymes arylsulfatase B and acid phosphatase, also found in the azurophilic granules of neutrophils. Lysophospholipase B (Charcot-Leiden crystals) - an enzyme in eosinophil membranes - does not play an important role in the pathogenesis of diseases and has no diagnostic value.
The functions of eosinophils are not precisely known. Eosinophils have many of the functions of other circulating phagocytes such as PMNs and monocytes. Although eosinophils are capable of phagocytosis, they kill bacteria within them less efficiently than neutrophils. There is no direct evidence that eosinophils kill parasites in vivo, but they are toxic to helminths in vitro, and helminth infections are often accompanied by eosinophilia. Eosinophils can modulate immediate hypersensitivity reactions by inactivating mediators released by mast cells (histamine, leukotrienes, lysophospholipids, and heparin). BOP and ECP are toxic to some parasites and mammalian cells. EN can seriously damage myelinated nerve fibers. BOP and ECP bind heparin and neutralize its anticoagulant activity. EPO in the presence of hydrogen peroxide and halogens generates oxidative radicals. Long-term eosinophilia sometimes leads to tissue damage, the mechanisms of which are not yet clear. The extent of damage is related to eosinophilic tissue infiltration, duration of eosinophilia, and degree of eosinophil activation. The greatest damaging effect of eosinophils was found in conditions like Churg-Strauss syndrome and idiopathic hypereosinophilic syndrome.
Eosinophils in a normal blood smear make up 1 to 5% of white blood cells. In absolute numbers, 50–250 eosinophils per 1 μl (50–250 × 106/l) of peripheral blood are considered normal. The critical level, which indicates a pathological process associated with an increase in the number of eosinophils, is a cell level exceeding 450 in 1 μl.
There are 3 degrees of eosinophilia: mild – 400–1500 cells per 1 µl, moderate – 1500–5000 cells per 1 µl, severe – more than 5000 cells per 1 µl. Many hematologists consider eosinophilia to be mild when there are 10–15% eosinophils in the peripheral blood; expressed if their number exceeds 15%; and conditions in which the number of eosinophils is more than 15–20% are proposed to be called “large blood eosinophilia.” They are usually combined with an increase in the total number of leukocytes.
The absolute number of eosinophils in peripheral blood in healthy people varies between 0–0.45 × 109/L. The number of eosinophils is inversely proportional to a person’s age (newborns have the most of them). Daily fluctuations in the number of eosinophils are inversely related to plasma cortisol levels, with a maximum occurring at night and a minimum in the morning.
In various clinical situations, activation of eosinophils can occur through different, as yet insufficiently studied mechanisms, and the results of this activation can be both protective (to a greater or lesser extent) in nature, for example, in helminthiasis, allergic diseases, and clearly pathological (in granulomatous processes ). Initially, in situations with normal reactivity, the main function of eosinophils is to limit allergic processes: eosinophils prevent their generalization by utilizing mediators of allergic inflammation and localizing the inflammatory response. However, in pathology, this measure of protection goes beyond the scope of biological expediency and begins to acquire the features of a particular disease [5].
Etiology and pathogenesis of eosinophilia
From the point of view of etiology, eosinophilia is divided into two large groups: idiopathic hypereosinophilic syndrome and reactive eosinophilia (Fig. 1). Reactive eosinophilia can have clonal (malignant) and non-clonal (secondary) characteristics [6].
The most common causes of nonclonal (reactive) eosinophilia (10−40% of the total number of leukocytes) are [6]:
- allergic diseases: bronchial asthma, hay fever, eczema, urticaria, angioedema, drug allergies, insect bites, serum sickness, etc.;
- immunopathological disorders: Omenn syndrome (a type of severe combined immunodeficiency), primary immunodeficiencies;
- skin diseases: scabies, toxicoderma, dermatitis herpetiformis, angioedema, pemphigus;
- parasitic diseases: ascariasis, trichinosis, echinococcosis, visceral form of larva migrans, strongyloidiasis, filariasis, malaria, toxoplasmosis, pneumocystosis;
- hematological diseases: lymphogranulomatosis, condition after splenectomy, Fanconi anemia, thrombocytopenia with absence of the radius (TAR syndrome), Kostmann syndrome (agranulocytosis of newborns - an autosomal recessive disease), infectious mononucleosis;
- familial hemophagocytic syndrome;
- familial eosinophilia;
- ionizing radiation and exposure;
- pulmonary eosinophilia: idiopathic acute eosinophilic pneumonia, idiopathic chronic eosinophilic pneumonia, eosinophilia with systemic manifestations and bronchial asthma (Churg-Strauss syndrome), tropical eosinophilia, allergic bronchopulmonary aspergillosis, Wegener's granulomatosis;
- gastrointestinal disorders: eosinophilic gastroenteritis, ulcerative colitis, protein-losing enteropathy, Crohn's disease;
- mixed group of pathological conditions: polyarteritis nodosa, metastatic disease, liver cirrhosis, peritoneal dialysis, chronic kidney disease, Goodpasture's syndrome, sarcoidosis, thymoma.
The currently known mechanisms for the formation of peripheral blood eosinophilia are antibody-dependent chemotaxis, which develops during parasitosis (IgE or IgG antibodies); immune, mediated through IgE (characteristic of allergies); response to eosinophilic chemotactic factor secreted by some tumors; tumor eosinophilia itself (leukemia) does not allow us to create a holistic understanding of the pathogenesis of this phenomenon in pathological processes of various origins [3, 4, 7].
Recent studies have shown that the eosinophil is one of the most aggressive effector cells of inflammation. Eosinophilic granules serve as a source of a large number of cytotoxic products, the increased content of which determines the formation of a high microbicidal potential, realized both against foreign substances and surrounding tissues [1, 8–11]. However, in modern literature, eosinophilic granulocytes are usually considered not only as an active participant in the development of allergic diseases and anthelmintic immunity, but also as an important factor in maintaining tissue and immunological homeostasis. Eosinophils have the ability to secrete a wide range of biologically active substances and express various receptor structures and adhesive molecules on their surface. Leukocytes of the eosinophilic series, due to the secretion of immunoregulatory molecules (interleukin-6 (IL-6), interleukin-10 (IL-10), interleukin-2 (IL-2), interferon-gamma (IFN-gamma)) participate in the regulation of the functions of immunocompetent cells. At the same time, eosinophils take part in the processes of phagocytosis, cellular repair, antigen presentation, inflammation, implementation of innate and acquired immunity, blood coagulation, etc. [4, 9, 10, 12].
The main source of cytokines in the body are helper T lymphocytes (Th). Activation of Th-1 lymphocytes producing IL-2, IL-3, IFN-gamma and tumor necrosis factor alpha (TNF-alpha) leads to the initiation of a cellular immune response. The formation of an immune response of the humoral type occurs under the dominant influence of Th-2 cytokines - interleukin-4 (IL-4), IL-5, IL-6, interleukin-9 (IL-9), etc. [13–19] . According to modern concepts, it is the imbalance in the production of Th-1/Th-2 cytokines that can have an important pathogenetic significance in the development of many diseases [20–24].
The processes of proliferation, differentiation and activation of eosinophilic leukocytes are particularly influenced by mediators produced predominantly by Th-2 lymphocytes [9, 25, 26]. Thus, the key mediator that modulates the functional activity of eosinophils is IL-5 (eosinophilopoietin), which belongs to the group of pro-inflammatory Th-2 cytokines. IL-5, originally called B-cell growth factor II, selectively stimulates the formation of eosinophils from their committed precursor colony-forming unit erythroid (CFU-E). IL-5, along with IL-3 and GM-CSF, activates their degranulation and release of cytotoxic proteins, regulates the expression of integrin molecules (CD11b, CD18), leading to an increase in circulating eosinophils, and by inhibiting the apoptotic death of eosinophilic leukocytes, prolongs their residence time in the bloodstream [9, 25, 27–29]. An antagonist of IL-5 in the regulation of the processes of programmed death of eosinophils is interleukin-12 (IL-12) [27, 30].
Chemokines and eotaxins secreted by endothelium, epithelium, monocytes and T lymphocytes bind to chemokine receptor-3 of eosinophils, which leads to migration of the latter into tissues. After activation, eosinophils express a high-affinity Fc receptor for IgE, IgG and complement components, the interaction of which promotes the release of toxic products and proinflammatory cytokines from eosinophil granules: peroxidase, collagenase, cationic protein, neurotoxin, leukotrienes, complement component C4, etc. Subsequently, eosinophils undergo apoptosis or necrosis and are phagocytosed by macrophages.
The absorption of apoptated eosinophils prevents the release of tissue toxic substances they contain. This also leads to the release of anti-inflammatory cytokines (transformed germ factor-beta, IL-10, prostaglandin E2) from macrophages. When eosinophils undergo necrosis, their tissue-toxic contents (cationic protein, enzymes, lipids, neurotoxins) are released. Phagocytosis of necrotic eosinophils by macrophages leads to the release of proinflammatory cytokines (thromboxane B2 and GM-CSF). For this reason, in the treatment of eosinophilia, drugs that induce eosinophil apoptosis (glucocorticosteroids, cyclosporine) are used [6].
Although eosinophils primarily exhibit two main functions—modulation of immediate hypersensitivity reactions and destruction of parasites (primarily helminths), persistence of eosinophilia in peripheral blood can lead to endothelial and endocardial damage due to intravascular degranulation of these cells. Cytolytic enzymes contained in eosinophil granules, damaging endothelial cells throughout the body, can cause the development of thrombosis or endocardial fibrosis [31].
Most often, patients with eosinophilia are identified in the practice of pulmonologists and allergists [3, 7, 10]. However, eosinophilia is not uncommon in diseases of the heart and blood vessels (systemic vasculitis) [22, 32]. Very often, the above syndrome occurs in patients with parasitic (opisthorchiasis, trichinosis, schistosomiasis, filariasis, etc.), fungal (aspergillosis) and viral (hepatitis A, B and C, infectious mononucleosis) diseases [2, 3, 23, 24, 33 –35]. In addition, hematologists and oncologists are often faced with the problem of interpreting the causes of eosinophilia syndrome in patients with neoplastic processes of the blood system originating from precursor cells of both lympho- and myelopoiesis (acute myeloid leukemia, lymphogranulomatosis, acute lymphoblastic leukemia, eosinophilic leukemia) [ 2, 3, 7, 36–38]. Taking medications can also lead to the development of eosinophilia [1, 7, 8, 10, 39–41]. Genetically determined forms of eosinophilia and an idiopathic variant of its occurrence have been described [1, 22, 37].
Clinical picture of IHES
Idiopathic hypereosinophilic syndrome is characterized by a long-term increase in the number of peripheral blood eosinophils and infiltration of many organs and tissues by eosinophils, which causes the clinical picture of multiorgan damage. IHES is a rare disease of unknown etiology, first described in 1968. The term “idiopathic hypereosinophilic syndrome” was proposed by Chesid et al. in 1975. Empirically, IGES includes:
- ongoing eosinophilia > 1.5 × 109/L for more than 6 months or death before 6 months associated with signs and symptoms of hypereosinophilic diseases;
- lack of evidence of parasitic, allergic, or other known causes of eosinophilia, despite comprehensive testing;
- suggestive signs and symptoms of organ involvement, including hepatosplenomegaly, organic heart murmurs, heart failure, diffuse central nervous system abnormalities, pulmonary fibrosis, fever, weight loss or anemia, and histologically proven eosinophilic infiltration of the affected organ or tissue or the presence of objective evidence of clinical pathology associated with eosinophilia, unless there is another identified cause [42].
Idiopathic hypereosinophilic syndrome is a diagnosis of exclusion and is made if the cause of IHES cannot be determined.
Recent studies have demonstrated that so-called IHES represents a significant group of heterogeneous disorders that may result from proliferation of lymphocytes or eosinophils themselves.
IHES occurs more often in men than in women (9:1), and begins between 20 and 50 years. This syndrome is also not uncommon in children. Boys get sick more often than girls, their ratio is 4:1. In children, IHES may be associated with trisomy 8 or 21 [6].
The clinical picture of the syndrome is manifested by such nonspecific symptoms as general malaise, anorexia, weight loss, periodic abdominal pain, night sweats, cough (usually nonproductive), muscle pain, angioedema, urticaria, and fever. The frequency of organ damage varies, hematological syndrome occurs in 100% of patients, heart damage - in 58%, skin manifestations - in 56%, nervous system damage - in 54%, pulmonary syndrome - in 49%, liver damage - in 30%, gastrointestinal symptoms – in 23% [43].
A key role in making a diagnosis is played by counting the number of leukocytes and determining the blood count. Thus, the number of leukocytes in some patients increases to 90,000 or more, pronounced eosinophilia is observed, accounting for more than 50% of the total number of leukocytes, which always prompts the exclusion of leukemia. The high content of white blood cells occurs in mature forms of eosinophils, but in some patients eosinophil precursor cells also appear. Examination of the bone marrow demonstrates its enrichment in both mature forms of eosinophils and their precursors. Chromosomal and cytogenetic changes in IHES have not been described.
Heart damage is considered an unfavorable prognostic sign, since this can cause disability, and in particularly severe forms of the pathological process, the direct cause of death of the patient. In the clinical picture, three phases of myocardial damage can be distinguished. The initial stage is described as the stage of acute necrosis; the intermittent stage, following the necrotic one, is characterized by the formation of intracardiac blood clots, which form at the site of previously developed necrosis; finally, the third stage is fibrotic.
The acute stage of myocardial necrosis occurs in the first month and a half from the development of hypereosinophilic syndrome. Damage to the endomyocardium occurs due to its infiltration by lymphocytes and eosinophils; from the granules of the latter a significant number of substances are released, leading to necrosis of cardiomyocytes and the formation of aseptic microabscesses of the myocardium. At this stage of the disease, clinical manifestations are minimal, therefore, only thromboembolism that has occurred and an active search for its source can reveal signs of myocardial damage resulting from eosinophilic infiltration of the endomyocardium and the development of a necrotic process. Initial manifestations of eosinophilic infiltration can be confirmed by endomyocardial biopsy, while echocardiography and other methods for diagnosing damaged myocardium are unspecific and insensitive. At the second stage, blood clots form on the damaged endocardium. The third stage is caused by the effect of eosinophil proteins on the endocardium and is characterized by progressive fibrosis involving the valves, shortening of the chordae, the formation of mitral and tricuspid insufficiency, and the development of restrictive cardiomyopathy. Clinical manifestations of this stage may include shortness of breath, pain in the heart region, left and right ventricular failure, and regurgitation sounds [43]. When conducting echocardiography, thickening of the cusps of the mitral and tricuspid valves, thickening of the endocardium, intracardiac thrombi are detected, and impairment of ventricular diastolic function is observed [44]. A high degree of atrioventricular block, manifested by syncope, has been described in a patient with local thinning of the interventricular septum detected during echocardiography [45].
Neurological symptoms in patients with IHES may occur due to cerebral thromboembolism, and may also manifest as clinical symptoms of encephalopathy or peripheral neuropathy. Cerebral thromboembolism occurs due to the introduction of a blood clot from the heart cavity and manifests itself in the form of a stroke or transient ischemic episodes. Anticoagulant therapy, as a rule, does not bring the desired effect, since embolisms can recur despite the therapy. Encephalopathy is manifested by changes in the sphere of consciousness, decreased memory, and the possible development of ataxia. In some patients, signs of damage to motor neurons appear, as evidenced by increasing muscle tone and a positive Babinski reflex. Peripheral neuropathy occurs in almost every second patient with IGES as a result of the toxic effect of eosinophilic proteins released during eosinophil degranulation, and manifests itself in the form of changes in sensitivity and muscle atrophy. The severity of damage to the peripheral nervous system varies from mild neuropathies to paraplegia with restoration of function during treatment with prednisolone [46].
Pathological changes in the skin are a fairly common clinical problem in patients with IHES. Patients are referred for angioedema, urticarial and erythematous rashes, the formation of itchy papules and nodules. The occurrence of skin symptoms is based on perivascular infiltration by eosinophils, and to a lesser extent by neutrophils. A biopsy reveals perivascular infiltrates containing eosinophils, neutrophils, mononuclear cells; there are no signs of vasculitis. Less common are ulcers of the mucous membranes of the nose, mouth, pharynx, esophagus, and stomach [47]. A biopsy reveals nonspecific changes in the form of mixed infiltrates without eosinophils, sometimes microthrombi. The development of skin symptoms, such as angioedema, urticarial rashes, is among the signs that indicate a favorable course of the disease. They regress quite quickly when glucocorticosteroid therapy is prescribed.
Patients with symptoms of rhinitis in hypereosinophilic syndrome may have nasal eosinophilia, polyps in the absence of an allergy history, negative skin tests, normal IgE levels and absence of aspirin intolerance.
The changes observed in the respiratory system are varied in their clinical manifestations. Patients often complain of a nonproductive cough and shortness of breath, but bronchial asthma is not a characteristic symptom for patients with IHES. X-ray examination reveals infiltrates in only 25% of patients resulting from the migration of eosinophils into the lung parenchyma. Pulmonary fibrosis may develop, especially in patients with endocardial fibrosis [43].
Eosinophilic gastritis, eosinophilic enterocolitis, chronic active hepatitis, eosinophilic cholangitis and Budd–Chiari syndrome due to hepatic vein obstruction are the result of eosinophilic gastrointestinal lesions [43].
Diagnosis of eosinophilia
As mentioned above, making a diagnosis of IHES is very difficult because IHES is a diagnosis of exclusion and is made when the cause of IHES cannot be determined. Therefore, according to the etiological classification, it is necessary to exclude reactive eosinophilia (clonal and non-clonal).
Considering the variety of pathologies in which eosinophilia is observed, as well as the severity of complications, a diagnostic algorithm has been developed for the differential diagnosis of reactive eosinophilia, which includes a number of laboratory and clinical instrumental studies.
When identifying mild and moderate eosinophilia, the following laboratory tests are used: stool analysis for the presence of cysts, eggs and fragments of parasites, determination of serological markers of parasitic infections, isohemagglutinin titer, IgM and IgE levels; exclusion of diffuse connective tissue diseases: detection of antinuclear antibodies, antibodies to double-stranded DNA, antineutrophil cytoplasmic antibodies (ANCA). To diagnose lesions of the digestive tract, serological markers of viral hepatitis and milk precipitin are determined; for the purpose of differential diagnosis with irritable bowel syndrome, the erythrocyte sedimentation rate (ESR) is determined, endoscopy, biopsy of the intestinal mucosa, and radiological examinations of the abdominal organs are performed; chest x-ray; biochemical blood test to determine liver and kidney functions.
In order to exclude possible organ damage, the examination includes echocardiography, electrocardiography, and determination of the concentration of cardiac troponin T in the blood serum. Some patients undergo a liver biopsy; Pulmonary function tests and bronchoalveolar lavage are examined; A neurological examination is carried out, including examination, electroencephalography, fundus examination, nerve conduction examination, and radiological examination of the brain.
For persistent severe eosinophilia, methods for determining clonality are used (immunophenotyping, cytogenetic examination of bone marrow) [48].
Differential diagnosis is carried out with parasitic and fungal diseases, acute eosinophilic leukemia, Churg-Strauss syndrome.
Treatment of IGES
The goals of treatment for patients with IHES are to increase the length and quality of life, achieve remission, reduce the risk of exacerbations, prevent irreversible damage to vital organs, and reduce the risk of developing side effects of treatment.
A patient with IHES is hospitalized to clarify the diagnosis, assess the prognosis and select treatment, as well as in case of exacerbation of the disease and the development of complications.
The treatment program consists of prescribing glucocorticosteroids (GCS), vincristine, hydroxyurea and interferon-alpha, which can slow the progression of the disease [8]. In addition, some studies have demonstrated the effectiveness of intravenous administration of antibodies to IL-5 (mepolizumab), which made it possible to reduce the dose of systemic corticosteroids. Cardiovascular complications may require intensive care and surgery.
In some cases, during treatment, consultation with related specialists is necessary. Thus, intensive therapy, any change in treatment requires consultation with a hematologist, the appearance or worsening of skin manifestations - a dermatologist, in the absence of the effect of treatment of neurological manifestations or the development of new ones, consultation with a neurologist is necessary, in the absence of dynamics of the pulmonary process or the presence of negative changes - a pulmonologist, in case of development or worsening damage to the heart - a cardiologist, if the condition of the ENT organs appears or worsens - an ENT doctor.
The duration of the patient's disability with IHES depends on the severity of the disease. In case of hospitalization, it can be 30–90 days. Further management of the patient and his outpatient monitoring should be carried out by specialists with experience in treating this disease. Even with active treatment, life-threatening conditions may develop that require urgent hospitalization, and the hospital must be multidisciplinary and its staff must have appropriate experience.
The prognosis for idiopathic hypereosinophilic syndrome is relatively favorable. The treatment provided can significantly reduce patient mortality. Modern clinical observations demonstrate a 10-year survival rate of more than 70% of patients.
Diagnostics
Clinical examination data, supported by the results of additional methods, help to understand what disease caused weakness in the legs. The list of diagnostic procedures is determined by a neurologist based on information obtained during a survey and neurological examination of the patient. The following tests may be performed:
- Blood tests.
In the hemogram, attention is paid to red blood counts, leukocyte count, and ESR. Biochemical analysis can detect hormonal or electrolyte imbalances, muscle enzymes, and specific antibodies. - Radiography.
The condition of the bone structures of the spine and skull can be assessed using conventional photographs taken in two projections. But the study has low resolution and does not detect changes in soft tissues. - Tomography.
CT is the preferred method for visualizing recent strokes, tumors, hematomas, and posterior fossa fractures. Diffuse pathology of the substance of the spinal cord and brain is more accurately determined by magnetic resonance imaging. - Myelography.
The patency of the cervical canal is determined by introducing a radiopaque contrast agent into the subarachnoid space. Myelography is indicated for intervertebral hernias, spinal injuries, and tumors. - Electroneuromyography.
Diseases accompanied by nerve conduction disorders are diagnosed using ENMG data. During the study, the peripheral nerve is stimulated with electrical impulses and the muscle response is subsequently recorded.
To identify vascular diseases, ultrasound angioscanning and rheovasography are prescribed. Endocrinopathies require ultrasound examination of the thyroid and parathyroid glands, adrenal glands; in persons with myasthenia gravis, the size of the thymus is assessed. Diagnosis of hereditary diseases is carried out using cytogenetic and molecular genetic tests.
Foot massage