Key Facts
- An estimated 6 to 7 million people worldwide, primarily in Latin America, are infected with Trypanosoma cruzi, the parasite that causes Chagas disease.
- In Latin America, the main mechanism of transmission of Trypanosoma cruzi to humans is contact with triatomine bugs.
- Other routes of transmission of this disease are: oral (through food), blood transfusion (transfusion of blood or blood products), vertical transmission from mother to child (congenital infection), organ transplantation and finally laboratory incidents.
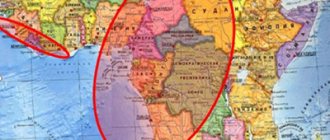
- Chagas disease was once confined to rural areas of the Region of the Americas, predominantly in Latin America, but in recent decades, as a result of population migration, most infected people live in urban areas (due to urbanization), and the disease has also spread on other continents (where the disease is no longer transmissible).
- Trypanosoma cruzi infection is curable if treated promptly.
- In the chronic stage, antiparasitic treatment can prevent or control the progression of the disease by preventing further transmission of the infection, for example from mother to child.
- Approximately 30% of people with chronic infection develop cardiac abnormalities, and 10% develop gastrointestinal, neurological, or combined disorders that may require specific treatment.
- The most effective way to prevent Chagas disease in Latin America is vector control and other measures aimed at preventing vector transmission of the disease.
- Worldwide, blood screening is critical to prevent infection from blood transfusions or organ transplants.
- It is also necessary to identify and treat this disease in girls and women of reproductive age, as well as to screen newborns and older children born to infected mothers who have not previously received antiparasitic treatment.
- Chagas disease is driven by a complex set of socioeconomic and environmental determinants and, given its many interrelated aspects, its management requires multisectoral approaches.
- People with Chagas disease are at risk of severe illness from COVID-19 and should be among the priority groups for vaccination.
Chagas disease, also known as American trypanosomiasis, is a potentially fatal disease caused by the protozoan parasite Trypanosoma cruzi.
It is estimated that Trypanosoma cruzi, the parasite that causes Chagas disease, infects 6 to 7 million people worldwide. The disease occurs predominantly in endemic areas of 21 countries in continental Latin America[1], where it is transmitted to humans and other mammals mainly through contact with waste products of triatomine bugs (the vectors of the disease), which are called differently in different geographical areas, in particular “kissing bugs”. bedbugs."
Chagas disease is named after Carlos Ribeiro Justiniano Chagas, a Brazilian physician and scientist who discovered this disease in 1909. In accordance with the decision of the 72nd World Health Assembly, World Chagas Disease Day was established in May 2021, which will be celebrated on 14 April (the day Carlos Chagas diagnosed the first human case of the disease in 1909, a two-year-old girl named Berenice).
Diseases caused by protozoa
Terms and Definitions
It’s a strange thing: it’s almost impossible to talk about the world of protozoa in a nutshell—you have to write a lot.
Contrary to their name, these microorganisms are very complex, diverse, inexhaustibly interesting, and sometimes unique. Some are deadly.
Previously, we learned about the existence of amoebas and ciliates in school zoology lessons (sometimes immediately forgetting: “I won’t need this in life, I’m going to be a programmer / top model / billionaire / DJ ...”). But lately this topic has somehow come up more and more often - for example, before a business or tourist trip to exotic countries, or in the context of a threat to pregnancy, or as a possible reason for thinness and lack of appetite in a child. We can still, anxiously looking at the clock in eternal time pressure, make hasty vaccinations “against everything” on the eve of departure to the tropics. Or, having learned from the news about another epidemic outbreak here, in your native country and hometown, to the eternal “We came here in large numbers...” irritably add: “... and brought all kinds of infection.”
But it’s not even about migration flows. The world itself has changed, becoming very small, cramped and round. There are no more distances. You have to learn about diseases that not every specialist knew before, and read about the causative agents of these infections, relying on the omnipotence of antibiotics and on your own immunity.
The immunity of Homo Sapiens, although strong, is not absolute; Moreover, we undermine it ourselves, we undermine it at every step, knowing full well what with. This is how our life is, and this is our civilization...
As for antibiotics, their unjustified prescription (by doctors) and amateur use (by patients) has long been a factor that deprives us of our last, perhaps, protection. “Antibiotics, if you take a loading dose, or better yet, two, kill any bacteria today,” this is what some Sapiens think, armed with penicillin and other, more modern and increasingly powerful generations of antibacterial drugs.
But protozoa are not bacteria. With them, everything is not so simple. They are called “protozoa” only in comparison with multicellular organisms, and even then it is not a fact that the latter are better adapted and more promising in terms of survival.
Questions about protozoal infections are becoming "increasingly asked" today. Let's try to understand at least the main points: how protozoa differ from bacteria, viruses and fungi; what diseases they can cause; why these diseases are classified as parasitoses in the group of infectious diseases.
The subkingdom of protozoa includes unicellular eukaryotes, i.e. microorganisms that have one or more cell nuclei. In this they differ from bacteria (prokaryotes, pre-nuclear and non-nuclear unicellular) and from viruses, which are a non-cellular form of organic matter (if we consider packaged clots of ribonucleic molecules as organic matter).
Most protozoa also differ:
- complex multiphase life cycle;
- the ability to reproduce both by asexual division with DNA recombination (like bacteria), and by more advanced, from a genetic point of view, gametogenesis (with the formation of pre-embryonic germ cells-gametes);
- the presence of organelles (pseudopods, flagella, cytostomes, cilia, membranes, etc.);
- heterotrophic nutrition (they can parasitize a macroorganism, absorbing its cells; or they feed on detrital, decaying organic matter, i.e. they lead a saprotrophic lifestyle; or they hunt and devour other microorganisms);
- the ability to both solitary and colonial existence;
- size (this characteristic varies widely among all the smallest, but on average protozoa are thousands of times larger than viruses and an order of magnitude larger than bacteria; some protozoa are visible to the naked eye, reaching several millimeters in size, and some giant multinuclear species grow up to 10-15 cm, remaining at the same time unicellular and meeting all the criteria of the protozoan subkingdom).
Being one of the oldest, primary forms of life (i.e., being part of the group of protist “predecessors”, which also includes lower fungi and algae), protozoan cultures are perfectly adapted in their ecological niche and at their level of biological hierarchy. As you know, evolution goes through all the possibilities and gives every chance to the strongest; the protozoan line chose to remain single-celled, having perfected non-bacterial methods of existence, movement, distribution, feeding, attack and self-preservation not inherent in other microorganisms over two billion years. Very diverse and not too closely related within their strange sub-kingdom, in general protozoa are animals - single-celled, but still animals - and in modern classifications of the earth's biosphere they are classified specifically in the animal kingdom.
There are over 30 thousand species of protozoa in four classes (sarcodaceae, flagellates, sporozoans, ciliates). Almost all of them are neutral in relation to humans. “Almost” – because there are exceptions: both asymptomatic parasitic and clearly pathogenic species are known. They have been studied by humans, of course, much better than other representatives (many of which have not been studied at all), but even here there is still a lot that is unclear. Due to the high adaptability of the pathogen and its ability to metamorphose (some stages of the life cycle are very stable and are specially designed to anabiotically “wait out” unfavorable conditions), protozoal parasitoses are, as a rule, severe and difficult to treat, and sometimes lightning-fast and almost completely lethal. These are, for example, amebic meningoencephalitis, in which the brain matter is corroded and absorbed by the “primitive” amoeba at an exponential rate.
Those who watched the four-part saga about “Aliens” probably still remember the nightmarish, from a human point of view, and in their own way perfect xenomorphs, which were extremely difficult to get rid of. If we reduce these creatures to hundredths of a millimeter and imagine them in the cellular space of living tissue, we will get a good idea of what happens in protozoal diseases. If it seems that the colors are too thick, let us take into account that protozoa kill several million people every year. Several million. Annually. And today this applies not only to distant countries.
Of course, there is no point in falling into dreary, doomed horror. Still, Homo Sapiens is immeasurably more complex (this is its weakness), more inventive and smarter - this is its strength. It is necessary to know, understand, exercise reasonable caution and take adequate measures. Only the most common protozoal parasitoses are listed and very briefly described below. Most of them are also covered in separate articles on the Lakhta Clinic website; Other diseases in this category, if necessary, will be considered in detail in the future.
Distribution area
Previously, the spread of Chagas disease was limited exclusively to rural areas of the continental Region of the Americas (excluding the Caribbean islands). However, mainly due to increased population mobility over recent decades, the majority of infected people now live in urban areas (due to urbanization), with the result that the disease is increasingly being detected in the United States of America, Canada, many European countries, and some countries in Africa, the Eastern Mediterranean and the Western Pacific.
Mechanisms of transmission
In Latin America, T. cruzi parasites are transmitted mainly through the infected waste products of blood-sucking triatomine bugs. Typically, these parasite carriers live in cracks in the walls or roofs of houses and domestic structures, such as chicken coops, livestock pens and storage buildings, in rural or suburban areas. During the day they usually hide, but at night they become active and feed on the blood of animals and humans. They typically bite exposed areas of skin, such as the face (hence the common name “kissing bugs”), and excrete waste products near the bite site. Parasites enter the human body when he instinctively rubs these secretions into the site of a bite or damage to the skin, eyes or mouth.
T. cruzi can also be transmitted by:
- by consuming T. cruzi-contaminated food or beverages contaminated with triatomine bugs or marsupials (foodborne outbreaks that infect large numbers of people at the same time typically have higher morbidity and mortality rates);
- during transfusion of blood or blood products received from infected donors;
- from an infected mother to a newborn child during pregnancy or childbirth;
- during transplantation of organs obtained from infected donors;
- as a result of laboratory incidents.
Signs and symptoms
Chagas disease has two stages. The first, acute stage lasts about two months after infection. During the acute stage, a large number of parasites circulate in the blood, but in most cases there are no symptoms of the disease or occur in a mild and erased form. In less than 50% of people bitten by triatomine bugs, the first visible signs may be skin lesions or purple swelling of the eyelids of one eye. In addition, you may experience fever, headache, swollen lymph nodes, pallor, muscle pain, shortness of breath, swelling, and pain in the abdomen or chest.
In the chronic stage of the disease, parasites are concentrated mainly in the heart or muscles of the digestive tract. Up to 30% of patients suffer from cardiac disorders and up to 10% from gastrointestinal (usually esophageal or colonic hypertrophy), neurological or combined disorders. In subsequent years, the infection can lead to sudden death due to cardiac arrhythmia or progressive heart failure caused by the destruction of the heart muscle and its innervation.
Patients with Chagas disease are at risk of severe illness from COVID-19 and should be included in priority groups for vaccination because SARS-CoV-2 infection can cause myocarditis and chronic Chagas disease usually leads to an increased risk of thrombosis, cardiac dysfunction and secondary ischemic strokes.
Publications in the media
Gastroesophageal reflux disease (GERD) is the development of inflammatory lesions of the distal part of the esophagus and/or characteristic symptoms due to repeated reflux of gastric and/or duodenal contents into the esophagus. Endoscopically positive gastroesophageal reflux disease—endoscopic examinations reveal reflux esophagitis. Endoscopically negative gastroesophageal reflux disease - there are no endoscopic manifestations of esophagitis.
Frequency. Symptoms of GERD are detected in almost half of the adult population, endoscopic signs - in more than 10% of people who have undergone endoscopic examination. Berrett's esophagus develops in 20% of patients with reflux esophagitis (0.4% of the population).
Etiology • Surgical interventions on or near the esophageal opening of the diaphragm: •• Vagotomy •• Resection of the cardia of the stomach •• Esophagogastrostomy •• Gastric resection •• Gastrectomy • Hiatal hernia • Peptic ulcer of the stomach and duodenum • Pylorospasm or pyloroduodenal stenosis • Scleroderma • Exogenous intoxications: •• Smoking •• Alcohol • Pregnancy • Drugs that can reduce the tone of the LES: •• Anticholinergic drugs •• B2-adrenergic receptor agonists and theophylline •• Calcium channel blockers and nitrates • Insufficiency of the cardiac sphincter in obesity.
Pathogenesis. Gastroesophageal reflux due to LES dysfunction. Variants and causes of dysfunction of the LES: • reduced tone of the LES at rest • prolonged or repeated transient relaxation of the LES • transient increase in intragastric and intra-abdominal pressure (with flatulence, constipation, obesity, pyloric spasm) • delayed gastric emptying • changes in esophageal motility • dilatation of the stomach • weakening mechanical factors supporting the antireflux barrier (diaphragmatic crura and cardioesophageal angle of His) • short esophagus.
Pathological anatomy • Changes are localized mainly in the distal part of the esophagus: •• limited (single erosions and ulcers without a tendency to merge) •• diffuse •• confluent, circularly covering the mucous membrane of the esophagus • In mild cases - moderate hyperemia and edema of the mucous membrane • In severe cases course - erosions, ulcers, scars, shortening of the esophagus, columnar cell metaplasia of the epithelium (Berrett's ulcer), longitudinal wrinkling of the esophagus (Berrett's syndrome) • In 8–10% of cases, ulcers become malignant.
Classification of reflux esophagitis • Grade A - one (or more) mucosal lesion less than 5 mm, limited to the mucosal fold • Grade B - one (or more) mucosal lesion more than 5 mm, limited to the mucosal fold • Grade C - one (or more) mucosal lesions extending to 2 or more mucosal folds but occupying less than 75% of the esophageal circumference • Grade D—one (or more) mucosal lesions extending to 75% or more of the esophageal circumference.
Clinical picture • Heartburn is the most characteristic symptom (83% of patients), appears as a result of prolonged contact of acidic (pH <4) gastric contents with the mucous membrane of the esophagus. Heartburn increases with errors in diet, drinking alcohol, carbonated drinks, physical stress, bending over and in a horizontal position • Belching increases after eating, drinking carbonated drinks • Regurgitation of food increases with physical stress and in a position conducive to regurgitation • Dysphagia of an intermittent nature, which is associated with hypermotor dyskinesia of the esophagus • Pain in the epigastrium (in the projection of the xiphoid process) or behind the sternum - appears soon after eating, intensifies when bending the body, in a horizontal position • Less commonly, odynophagia occurs, a feeling of a lump in the throat when swallowing, pain in the ear and lower jaw, chest pain that can be provoked by physical activity • Extraesophageal manifestations of GERD - chronic cough, pneumonia, dysphonia, broncho-obstruction, dental erosion, etc. - are caused by the ingress of gastric contents onto neighboring organs and the vagal reflex between the esophagus and the lungs.
Diagnostics • X-ray examination lying on the back or in an upright position with a strong anterior tilt of the patient: reflux of barium sulfate into the distal parts of the esophagus, X-ray picture of esophagitis • Endoscopic examination with biopsy: reflux esophagitis of varying severity, prolapse of the gastric mucosa into the esophagus, true shortening esophagus, reflux of gastric and/or duodenal contents into the esophagus. In the case of endoscopically negative GERD, there are no signs of esophagitis • Esophagotonokymography (manometry) - decrease in expiratory sphincter pressure, destructuring of the sphincter, increase in the number of transient relaxations of the LES, decrease in the amplitude of peristaltic contractions of the thoracic esophagus • Daily pH-metry - the main method for diagnosing GERD and monitoring the effectiveness of treatment . It is recommended to evaluate: the total time of reduced acidity with pH<4 (the most significant criterion) in a standing and lying position; total number of refluxes per day; number of reflux lasting more than 5 minutes; duration of the longest reflux • Bilimetry is performed to identify alkaline (bile) reflux • Scintigraphy is indicated to identify motor-evacuation disorders of the esophagus • Omeprazole test - clinical symptoms of GERD are significantly reduced within 3-5 days of daily intake of 40 mg omeprazole • Bernstein test - if present GERD injection of 0.1 N HCl solution into the esophagus leads to the appearance of clinical symptoms, incl. and in the case of endoscopically negative GERD.
TREATMENT
Non-drug measures • Lifestyle changes •• Smoking cessation •• Normalization of body weight •• Raising the head end of the bed •• Avoid stress on the abdominal muscles, bending the body, wearing tight belts • It is undesirable to take drugs that reduce the tone of the LES (nitrates) , calcium antagonists, theophylline, progesterone, antidepressants) • Diet: limiting foods that increase gas formation, spicy, very hot or cold foods; avoid drinking alcohol, foods that reduce the tone of the LES (onions, garlic, pepper, coffee, chocolate, etc.); Avoid overeating, last meal no later than 3-4 hours before bedtime.
Drug therapy is carried out for at least 8–12 weeks, followed by maintenance therapy for 6–12 months • Antacids and alginates •• Usually prescribed 1.5–2 hours after meals and at night •• Effective in the treatment of moderate and infrequent symptoms • Prokinetics - domperidone, metoclopromide, cisapride 10 mg 4 times a day • Proton pump inhibitors (omeprazole, lansoprazole, rabeprazole) in regular or double dosage. In cases of severe esophagitis, they are usually required to be prescribed in combination with prokinetics.
Surgical treatment • Indications for surgical treatment •• Complications of GERD (esophageal strictures, recurrent bleeding, Berrett's esophagus) •• Ineffectiveness of drug therapy in young patients •• Combination of GERD with bronchial asthma, refractory to adequate antireflux therapy • Antireflux operations, such as Nissen fundoplication .
Complications • Benign stricture of the esophagus • Ulceration of the esophagus • Bleeding from latent to profuse from the ulcerated mucous membrane of the esophagus • Cicatricial changes in the esophagus, up to its shortening and stenosis • Laryngospasm • Pulmonary aspiration • Berrett's esophagus.
Abbreviations. GERD - gastroesophageal reflux disease; LES - lower esophageal sphincter
ICD-10 • K21 Gastroesophageal reflux
Appendix • Berrett's syndrome is a chronic peptic ulcer of the lower esophagus with an epithelium resembling that of the cardial mucosa; esophageal stricture, most often develops as a result of gastroesophageal reflux “Berretta's esophagus • Berretta's esophagus is a complication of GERD in the form of metaplasia of the mucous membrane of the distal esophagus into the small intestinal epithelium. Berrett's esophagus is considered a precancerous condition • Schatzki rings - membranes of the mucous membrane in the lower third of the esophagus, narrowing or closing its lumen, of unknown etiology; the disease is more common in patients with gastroesophageal reflux. Diagnosis - X-ray contrast study using barium sulfate confirms the diagnosis when constriction is detected in the lower part of the esophagus. Treatment is esophageal dilatation and antireflux surgery. The course is chronic, progressive. Synonym. Ring syndrome in the lower part of the esophagus. ICD-10. Q39.4 Esophageal membrane.
Treatment
Benznidazole and nifurtimox are prescribed to kill the parasite that causes Chagas disease. The effectiveness of treatment with both drugs is almost 100% if treatment is started soon after infection at the onset of the acute stage, including in the case of congenital infection. However, the longer the infection, the less effective these drugs are, and older people are more likely to experience adverse drug reactions. Treatment is also prescribed to people with reactivated infection (for example, as a result of decreased immunity) and patients at the beginning of the chronic stage of the disease, including women and girls of reproductive age (before or after pregnancy) to prevent transmission of the disease to the child.
Infected adults, especially those who are asymptomatic, should be treated because antiparasitic therapy can also prevent or delay progression of the disease. In other cases, a balanced approach must be taken, taking into account both the potential benefits of drug treatment to prevent or delay the progression of Chagas disease, the duration of treatment (up to 2 months) and the risk of adverse reactions (which affect up to 40% of adult patients). Benznidazole and nifutrimox are contraindicated during pregnancy and in persons with renal or hepatic impairment. Nifurtimox is also contraindicated in people with a history of neurological or psychiatric disorders. In addition, specific treatment for cardiac, gastrointestinal, or neurological disorders may be indicated.
Diagnostic and treatment methods
In order to clarify the diagnosis, patients are prescribed:
- X-ray of the chest and abdominal organs;
- electrocardiogram;
- Echokg;
- endoscopic examination.
Treatment is aimed at destroying the parasite and eliminating life-threatening symptoms. In the acute phase of Chagas disease, two drugs are used: nifurtimox and benznidazole. Both drugs are supplied to regions with a high incidence of disease. In the USA, they are obtained exclusively through specialized centers involved in monitoring the epidemiological situation.
When the disease becomes chronic, these drugs are no longer effective. Narcotic analgesics help relieve painful symptoms, but they can only be used for young and middle-aged patients - no older than 50 years. The following may be used as additional measures:
- correct tactics for treating cardiovascular complications. For example, a patient with heart failure is sometimes helped by a pacemaker or, in extreme cases, by a heart transplant;
- treatment of complications from the digestive system. Here, too, surgical methods can be used, as well as diet, hormonal drugs and other types of medications.
Control and prevention
Initially (more than 9,000 years ago), T. cruzi parasites infected only wild mammals. This infection later spread among domestic animals and people. The presence of a large reservoir of T. cruzi parasites among wild animals in the Americas makes its complete eradication impossible. Thus, control of the parasite comes down to eliminating its transmission and ensuring timely access to medical care for those infected.
There is no vaccine against Chagas disease. T. cruzi can infect several species of triatomine bugs, most of which are endemic to the Americas. The most effective way to prevent Chagas disease in Latin America is vector control. To prevent infection from blood transfusions or organ transplants, and to better diagnose and care for affected populations, donated blood must be screened.
Depending on the geographic area, WHO recommends the following infection prevention and control approaches:
- spraying residual insecticides in residential premises and local areas;
- modernization of housing and improvement of sanitary and hygienic conditions to prevent infestation of residential premises by parasites;
- use of personal preventive measures, such as the use of bed nets and proper hygiene when preparing, transporting, storing and consuming food;
- Conducting locally adapted information, training and communication activities and preparing information materials on prevention and surveillance measures for different target populations and situations;
- blood donor screening;
- testing of donors and recipients of organs, tissues and cells;
- ensuring access to diagnosis and treatment for persons for whom antiparasitic treatment is recommended or indicated, especially children and women of childbearing age before pregnancy; And
- screening of newborns and older children born to infected mothers who have not previously undergone antiparasitic therapy to ensure timely diagnosis and treatment.
It is estimated that the cost of providing care to patients with cardiac, gastrointestinal, neurological, or combined disease is more than 80% higher than the cost of residual insecticide treatments for vector control and infection prevention.