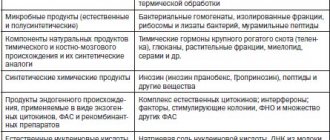
Doxorubicin (other names: Adriamycin, Rubex) is a chemotherapy drug from the group of anthracycline antibiotics. Along with it, this group includes: Epirubicin, Idarubicin, Daunorubicin. It is obtained from bacteria of the genus Streptomycetes, namely Streptomyces peuceticus var. caesius. Doxorubicin is used to treat various cancers. The drug solution is a red liquid. This drug and its side effects are familiar to women who have undergone red chemotherapy for breast cancer.
- How does Doxorubicin destroy tumor cells?
- What types of cancer can this drug be used for?
- Course of treatment with Doxorubicin
- Possible side effects
- Rare dangerous side effects
- Why is Doxorubicin a “red” chemotherapy?
- How is the patient's condition monitored during treatment?
- Pregnancy and Fertility
How does Doxorubicin destroy tumor cells?
All chemotherapy drugs destroy cancer cells or suppress their proliferation, but they do this in different ways. Doxorubicin is an antitumor antibiotic with antiproliferative and antimitotic effects. Its antitumor effects are associated with the following mechanisms:
- The drug penetrates the DNA molecule, blocks its replication and protein synthesis.
- Doxorubicin inhibits the enzyme DNA topoisomerase II, which affects the spatial structure of DNA and plays an important role in the process of cell growth and division.
- Chemotherapy molecules are capable of oxidizing to form free radicals that damage the cancer cell.
All these effects apply not only to tumor cells, but also to healthy cells. Doxorubicin can cause serious side effects. We will talk about them below.
Pharmacological properties of the drug Doxorubicin
Antitumor anthracycline antibiotic obtained from Streptomyces peucetius var. caesius . The mechanism of action is due to the ability to interact with DNA and interfere with the synthesis of nucleic acids. Reduces mitotic activity and causes chromosomal aberrations. Has a pronounced immunosuppressive effect. Highly active against many forms of tumors. It has immunosuppressive activity, cardiotoxicity, and inhibits hematopoiesis. Ineffective when taken orally. After intravenous administration, the concentration of doxorubicin in the blood plasma decreases rapidly, which is likely due to its distribution in the tissues of the body. Doxorubicin does not penetrate the BBB and is excreted mainly in bile and feces (within 7 days - 40–50% of the administered dose). About 5% of the administered dose is excreted in the urine within 5 days. If liver function is impaired, the removal of doxorubicin from the body slows down, resulting in its accumulation in the blood plasma and tissues.
What types of cancer can this drug be used for?
Doxorubicin is used for cancer of the breast, stomach, uterus, head and neck, kidney, liver, ovaries, and thyroid gland. It is also used to treat acute lymphoblastic and myeloblastic leukemia, bone sarcomas, Hodgkin and non-Hodgkin lymphomas, multiple myeloma, neuroblastoma, and soft tissue sarcoma.
Doxorubicin is usually prescribed in combination with other chemotherapy drugs that have different mechanisms of action. This increases the effectiveness of treatment.
Doxorubicin: contribution to modern antitumor therapy
Over the past 30 years, more than 2000 different drugs with antitumor effects have been created. Clinical studies of many of these drugs are ongoing, but doxorubicin, developed more than 40 years ago, remains the most widely used of all anthracyclines and one of the most commonly used cytotoxic drugs.
Doxorubicin was first isolated from the fungus Streptomyces peucetius through research conducted by Farmitalia Research Laboratories in the early 1960s. In 1974, doxorubicin was registered for clinical use in the USA [1, 2]. Subsequently, repeated attempts were made to modify the drug, but doxorubicin derivatives were inferior to it in terms of antitumor activity.
Doxorubicin is a class I anthracycline antibiotic that equally inhibits DNA and RNA synthesis. Cells in the S phase of the cell cycle are most sensitive to the drug. There are two main mechanisms of action of the drug that cause tumor cell death.
- Intercalation. Doxorubicin is inserted between two adjacent nucleotides, forming a strong interaction between DNA and the anthracycline ring, thereby disrupting DNA synthesis and transcription.
- Enzyme inhibition. Doxorubicin binds and inhibits topoisomerase II, one of the key enzymes in DNA synthesis.
In addition, as a result of drug metabolism, free oxygen radicals are produced, damaging DNA and disrupting the synthesis of the latter [1, 2].
Absorption of the drug is high, distribution occurs evenly. Doxorubicin does not cross the blood-brain barrier. Plasma protein binding is 74–76%.
Doxorubicin is metabolized in hepatocytes to form the active metabolite doxorubicinol. Enzymatic reduction of doxorubicin under the action of intracellular oxidases, reductases and dehydrogenases leads to the formation of free radicals, which causes the cardiotoxicity of the drug. After intravenous administration, the substance quickly disappears from the blood serum, concentrating in the liver, kidneys, myocardium, spleen, and lungs. The half-life (T1/2) for doxorubicin and doxorubicinol is 20–48 hours. Excretion: 40% of the drug is excreted unchanged in bile within 5 days, 5–12% (including metabolites) in urine within 5 days.
Doxorubicin (including Doxorubicin-Ebeve) is one of the most commonly used antitumor drugs. The drug is active both against solid epithelial and non-epithelial tumors, and against some myeloproliferative diseases.
Indications for the use of Doxorubicin-Ebewe are almost all malignant neoplasms: breast cancer, lung cancer, mesothelioma, esophageal cancer, stomach cancer, hepatocellular cancer, pancreatic cancer, insulinoma, carcinoid, head and neck cancer, thyroid cancer, malignant thymoma, ovarian cancer, testicular germ cell tumors, trophoblastic tumors, prostate cancer, bladder cancer, endometrial cancer, cervical cancer, uterine sarcoma, Ewing sarcoma, rhabdomyosarcoma, neuroblastoma, Wilms tumor, osteogenic sarcoma, soft tissue sarcoma, Kaposi sarcoma [1, 2].
The first reports about doxorubicin appeared in the scientific literature back in 1972, when active clinical studies of the drug in the treatment of malignant tumors began. One of the first studies was conducted on the effectiveness of doxorubicin in monotherapy for metastatic breast cancer. The objective response to treatment with doxorubicin was compared with the results achieved with the use of cytostatics, which were the standard treatment for breast cancer in those years - cyclophosphamide, methotrexate, 5-fluorouracil. The objective response rate in the group of patients receiving doxorubicin was 36% (44 of 121); when using cyclophosphamide, methotrexate, 5-fluorouracil - 34, 33, 26%, respectively. In addition, comparisons were made with a group in which combinations of standard cytostatics were used. The effectiveness of doxorubicin in monotherapy was similar to various combinations of cytostatics [4].
At the same time, a study was conducted to compare the effectiveness of doxorubicin in monotherapy and the five-component “Cooper regimen” (cyclophosphamide, methotrexate, 5-fluorouracil, vincristine, prednisolone), used in intermittent and continuous regimens for the treatment of metastatic breast cancer. The effectiveness of doxorubicin was found to be 55%, compared with 59% with the intermittent version of the Cooper regimen and 65% with the continuous version. Thus, the therapeutic potential of doxorubicin turned out to be similar to the five-component regimen, while the latter is characterized by significant toxicity [4].
The high antitumor activity of the new drug prompted researchers to develop and study drug combinations based on doxorubicin.
In the randomized phase II study E2104, breast cancer patients were divided into 2 groups depending on the presence or absence of bevacizumab as part of the treatment regimen, with the following regimen being the basic regimen: doxorubicin 60 mg/m2 + cyclophosphamide 600 mg/m2 every 14 days for 4 cycles followed by taxane therapy. Undoubtedly, the primary goal of this study was to determine the effectiveness and toxicity of bevacizumab, however, the relevance of doxorubicin in the treatment of breast cancer remained undeniable [19].
Clinical studies of doxorubicin were actively carried out in other tumor diseases. One of the first original studies to be published was a large phase II study of chemotherapy for solid and non-solid tumors with two doses of the drug - 60 and 75 mg/m2. It turned out that doxorubicin showed the greatest activity in the treatment of solid tumors in sarcomas [3]. In Ewing's sarcoma, doxorubicin activity was 48% (11/29); slightly less activity was observed in the treatment of osteogenic sarcomas - 35% (11/35); in the treatment of soft tissue sarcomas, the objective response rate was 30–36% [5]. The combination of chemotherapy with surgical treatment and radiation therapy gave the most effective result - a high median time to tumor progression (47 months; with a minimum of 16 months and a maximum of 82 months) [5, 6].
A little later, doxorubicin was first studied in the treatment of lung cancer. The drug was used in monotherapy, and the objective response obtained from data analysis was compared with the results of the most common drugs used to treat lung cancer at that time - lomustine, cyclophosphamide, methotrexate, hexamethylmelamine, mechlorethamine. The objective response rate when using these drugs in monotherapy was 27, 23, 25, 20, 36%, respectively. The objective response rate in the group of patients (n = 226) treated with doxorubicin was 26%, which is comparable to the result obtained in other groups [7].
Several subsequent clinical studies have described the activity of doxorubicin in combination with other drugs (etoposide, vincristine, cyclophosphamide, cisplatin) against disseminated small cell and non-small cell lung cancer. All doxorubicin-based drug combinations demonstrated higher objective response rates and a slight increase in time to tumor progression compared with doxorubicin monotherapy [8, 9].
Several clinical studies have demonstrated the effectiveness of doxorubicin in the treatment of diffuse pleural mesothelioma. The objective effect rate was 15%. The use of doxorubicin in combination with other drugs has increased patient survival compared to standard chemoradiotherapy [10, 11].
In combination with etoposide and cisplatin, doxorubicin has been used in the chemotherapy of disseminated and locally advanced gastric cancer. In a study examining the effectiveness of preoperative chemotherapy for locally advanced and unresectable gastric cancer, doxorubicin + etoposide + cisplatin was treated. The objective response rate was 70% (23/33), and the complete regression rate was 21% (7/33). 19 patients who had an objective effect underwent surgical treatment. The median survival was 18 months, and in the group of patients with complete tumor regression confirmed morphologically, the median survival was 24 months [12].
A multicenter phase II study also showed the high effectiveness of the combination of doxorubicin + etoposide + cisplatin in patients with metastatic gastric cancer. The objective effect was 64%, the rate of complete regression was 21%. Median survival for all patients was 9 months. In the group of patients who achieved complete regression, survival increased to 17 months, and among patients with complete morphological regression, the median survival was 23 months [13].
The activity of doxorubicin against tumors of the female reproductive system was demonstrated in a study of the effectiveness of the drug in combination with cisplatin and cyclophosphamide in the treatment of ovarian cancer compared with the combination without doxorubicin. The phase III study included 125 patients who had previously undergone surgery.
In the group of patients receiving doxorubicin as part of the combination, there was a significant increase in the objective effect rate - 56.2% (40.6% complete regression) compared with 46% in the group of patients receiving cisplatin and cyclophosphamide, as well as a slight increase in overall survival [ 14].
JE Ang et al. published data from a study of doxorubicin in chemotherapy for tumors of the female reproductive system, in which patients with advanced or recurrent endometrial cancer and carcinosarcoma received a so-called doublet regimen: four cycles of carboplatin (AUC-5) and doxorubicin (50 mg/m2) + four cycles of carboplatin (AUC -5) and paclitaxel (175 mg/m2) at 21-day intervals. High treatment results were obtained: an objective response was recorded in 82.1 and 66.7% of patients with endometrial cancer and carcinosarcoma, respectively. At a mean follow-up of 21 months, the median time to progression was 15.3 and 12.0 months for patients with endometrial cancer and carcinosarcoma, respectively. It was shown that this regimen was not accompanied by increased toxicity and was satisfactorily tolerated by patients [18].
Doxorubicin also has activity in hormone-refractory prostate cancer. The study included 25 patients who were administered weekly doxorubicin at a dose of 20 mg/m2. The observed objective effect was 84% [15].
The effectiveness of doxorubicin, like other antitumor agents, was one of the first to be studied in the treatment of metastatic bladder cancer. The objective response to doxorubicin treatment was 33% (15/52). At that time, only 5-fluorouracil had similar proven effectiveness (objective response 35%) [16].
In addition to the systemic use of doxorubicin in the treatment of bladder cancer, intravesical administration of the drug in adjuvant therapy is also possible. Doxorubicin was studied in combination with cisplatin as a chemotherapeutic agent in the treatment of endometrial cancer (1991). This approach demonstrated the greatest objective effect [17].
Kaposi's sarcoma must be added to the list of nosologies for the treatment of which doxorubicin is used. The results of studies on the effectiveness of the drug in the treatment of this disease were first published in 1984.
In the treatment of hemoblastoses, the use of the drug is indicated for acute lymphoblastic leukemia, acute myeloblastic leukemia, chronic lymphocytic leukemia, Hodgkin's disease and non-Hodgkin's lymphomas, multiple myeloma.
It is worth noting that the relatively low cost of doxorubicin in comparison with other drugs allows it to be widely used in modern oncological practice. Thus, according to a literary analysis by HA Azim et al., when treating patients with non-Hodgkin's lymphoma, the addition of rituximab to standard chemotherapy in the regimen of cyclophosphamide + vinorelbine + adriamycin + prednisolone improved the results of therapy, but for economic reasons, the use of rituximab was not always possible. In such cases, according to studies from the 1990s, it is the dose intensity of doxorubicin as part of various treatment regimens that is the key factor in increasing patient survival, the review emphasizes [20].
Thus, research on doxorubicin played a huge role in the development of various chemotherapy regimens for solid tumors, while the drug still does not lose its relevance in the treatment of many nosologies.
Doxorubicin-Ebeve has moderate toxicity, which is effectively controlled.
Of the main side effects of the drug, the following should be noted.
On the part of the hematopoietic organs, leukopenia and neutropenia are possible (the peak of neutropenia occurs on the 10th–14th day of the cycle); Thrombocytopenia and anemia occur less frequently.
From the cardiovascular system, sinus tachycardia and nonspecific changes in ST-T waves are possible as early cardiotoxicity; tachyarrhythmia, ventricular tachycardia, ventricular extrasystole, atrioventricular block, and bundle branch block are also possible.
Late manifestations of myocardial toxicity may be a decrease in left ventricular ejection fraction with or without manifestations of congestive heart failure. Free radicals formed as a result of the metabolism of doxorubicin cause damage to the actin-myosin complex of muscle fibers with the subsequent development of dilated cardiomyopathy.
From the digestive system, the toxicity of the drug can manifest itself as anorexia, nausea, vomiting, stomatitis, esophagitis, diarrhea, colitis. It is also possible to increase the level of total bilirubin and liver enzymes.
For the skin and skin appendages, the main side effect is complete reversible alopecia.
In conclusion, it should be noted that today doxorubicin remains one of the priority drugs in the treatment of many tumor diseases. Doxorubicin has become the basis for various combinations of anticancer drugs, many of which are standard of care for solid tumors.
Course of treatment with Doxorubicin
Doxorubicin is administered intravenously in different ways:
- Through an IV.
- Using an infusion pump - for a long time at a certain speed.
- Through a central venous catheter installed, for example, in the subclavian vein.
- Through an infusion venous port system: a small reservoir is sutured under the skin, connected by a catheter to the subclavian vein. One of the walls of the reservoir is a membrane, it is located directly under the skin. The drug is injected into the reservoir by piercing the skin and membrane with a special needle. Typically, a patient is implanted with a port system when drugs need to be administered over a long period of time.
- Sometimes, for bladder cancer, Doxorubicin is injected directly into the bladder through a catheter.
The drug is not available in tablets and is not used.
IMPORTANT. Doxorubicin is a vesicant and has a strong blister effect. If you inject it into the subcutaneous fatty tissue, it will cause necrosis - tissue death. This is a serious complication; after unsuccessful administration of the drug, skin grafting may be required. Therefore, the procedure must be performed by specially trained medical workers, in compliance with all rules. If redness appears on the skin during administration, you should immediately inform your doctor or nurse.
Therapy can be carried out both in a hospital and on an outpatient basis. The dosage of Doxorubicin depends on the type of cancer, the patient’s weight and height, his state of health, and concomitant diseases. Treatment, as with other chemotherapy drugs, is carried out in cycles. After the administration of Doxorubicin, there should be a “respite” for several days - this is necessary so that the body can recover and no serious side effects arise. Then the cycle is repeated. The course may consist of several cycles and last several months.
When is chemotherapy indicated?
The decision on drug treatment of oncology is made by the attending physician, based on the results of tests and hardware studies. As a rule, chemotherapy is used in the following cases:
- Type of cancer. The choice of surgical or conservative treatment depends on the type of malignant tumor, its size and stage of development. Chemotherapy is always given after surgery to destroy remaining cancer cells and possible metastases.
- Features of the patient's body. If a cancer patient is elderly and most likely will not survive the operation, or for other reasons (the tumor is localized in a place inaccessible to surgery, poor general condition, chronic diseases), he is prescribed chemotherapy as treatment. In this case, the emphasis is on inhibiting the growth of cancer cells and increasing the life expectancy of a person with cancer.
Most often, drug therapy for oncology is prescribed to prevent metastasis of previously removed tumors, as well as to treat blood cancer (leukemia, hemoblastosis) and other rare types of malignant neoplasms (choriocarcinoma, rhabdomyosarcoma). Chemotherapy is also used to prepare the tumor for surgery so that the surgeon can completely remove it without leaving a single cancer cell.
Possible side effects
Like any chemotherapy drugs, Doxorubicin damages DNA not only in tumor cells, but also in other actively reproducing cells. Because of this, it may cause some side effects.
But do not be alarmed if your doctor has prescribed this drug to you. Firstly, you will not immediately experience the entire list of side effects that we list below. Secondly, most of them are quite predictable in terms of time of onset, severity and duration. After completion of treatment, they gradually disappear. Thirdly, the side effects of Doxorubicin can be kept under control, and Euroonko has everything necessary for this.
Common side effects include: nausea and vomiting (beginning to occur approximately 2 weeks after starting treatment), pain at the injection site, hair loss, anemia and an increased risk of infection. More than a third of patients experience these symptoms. 10–30% of patients experience symptoms such as mouth ulcers, watery eyes, darkening of the skin at the site of radiation therapy, and red urine. Infertility occurs in 10% of cases in men and women.
Decreased immunity. When using Doxorubicin, the level of leukocytes in the blood decreases and the risk of infection increases. You should immediately inform your doctor about symptoms such as fever over 37.5 °C, sore throat, cough, loose stools, and frequent urination.
Increased bleeding. Occurs due to a decrease in platelet levels. It manifests itself in the form of hematomas (bruises) on the skin, bleeding gums, and nosebleeds. Some patients may require platelet transfusions.
Anemia. Chemotherapy with Doxorubicin can lead to a decrease in the level of red blood cells in the blood. There is pallor, a constant feeling of fatigue, headaches and dizziness, shortness of breath. Some patients require red blood cell transfusions.
Increased fatigue. This symptom worsens toward the end of chemotherapy and persists for several weeks after completion. At this time, you need to try to relax more and be in the fresh air.
Changes in the oral mucosa. The oral mucosa may become sensitive and painful, and sometimes ulcers appear on it. Increased risk of infection. In order to cope with these symptoms during treatment with Doxorubicin, you need to follow some recommendations:
- Use a soft toothbrush.
- Drink more fluids.
- Avoid eating foods that irritate the mucous membranes of your mouth.
- Stop drinking alcohol and smoking.
Decreased appetite. This is a common side effect of Doxorubicin and other chemotherapy drugs. If you eat very little for several days due to lack of appetite, consult your doctor.
Pink-red coloration of urine. Urine may change color within 48 hours after using the drug. There's nothing wrong with that. This is not blood, this symptom occurs because the Doxorubicin solution is red.
Diarrhea. If the stool becomes liquid, you should immediately consult a doctor. This symptom may be due to the drug affecting the intestines or an infection.
Hair loss. Hair may become thin or fall out completely, and not only on the head: many patients lose eyebrows and eyelashes. Most often this occurs after the first or second course of treatment with Doxorubicin. For many patients, this side effect seems the most terrible, but in fact there is no reason to panic. Typically, hair grows back soon after chemotherapy is completed. In order to protect them, special cooling caps are used during procedures.
Changes in skin and nails. Nails may become brittle, skin may become dry, become more sensitive to the sun, and dark spots may appear. Usually these symptoms are temporary.
Other side effects are possible, but these can usually be managed successfully. Doctor's recommendations and modern methods of supportive treatment help patients comfortably tolerate Doxorubicin chemotherapy.
Special instructions for the use of Doxorubicin
Strict hematological and cardiac monitoring is necessary, peripheral blood composition and ECG studies should be performed before starting therapy and before each subsequent course. Doxorubicin is widely used in complex polychemotherapy regimens, but it should not be prescribed earlier than 1 month after completion of the previous cycle of chemotherapy using other antitumor agents. The antitumor activity of doxorubicin is approximately the same regardless of the administration schedule - whether it is administered as a bolus (total dose) once a month, weekly or by gradual continuous administration, however, different administration regimens differ significantly in the severity of adverse reactions. In particular, with prolonged administration, in contrast to bolus administration, nausea and vomiting are significantly less pronounced.
Rare dangerous side effects
An extremely rare but very serious side effect of Doxorubicin is a decrease in the pumping function of the heart. The risks are increased in elderly patients, people receiving radiation therapy to the chest area, and taking other drugs with cardiotoxic effects. Heart problems may occur several years after treatment has been completed. Before starting a course of chemotherapy, the doctor must prescribe an ultrasound and other tests and monitor the patient’s heart condition during the treatment process.
Sometimes tumor decay syndrome occurs - a condition when, under the influence of the drug, many tumor cells die, and the products of their destruction enter the blood. Symptoms appear within 24–48 hours after administration of the drug, and kidney failure may occur. To prevent this complication, infusion therapy is prescribed.
Another extremely rare complication is leukemia. It may occur several years after treatment.
Additional information about the use of Doxorubicin
If doxorubicin gets on the skin around the injection site, it can cause damage, including necrosis. If the patient feels a tingling or burning sensation around the vein during the infusion, or if the drug spills from the injection tube onto the skin, contact a nurse or doctor immediately. If the injection site becomes red or swollen, this should also be reported immediately, even if this phenomenon was discovered at home and not in the hospital room. There are also people who experience facial redness and heat during the infusion.
Because other drugs may interact with doxorubicin, you should tell your doctor about all medications you are taking, either prescription or on your own.
View all reviews
A patient with stage four lung cancer was cured at the Imedical Medical Center!
After I received help at the Imedical medical center, I became convinced from my own experience that Israel is the best choice for treating cancer, while in Russia cancer is still a death sentence for patients. Belov Andrey, 40 years old (Moscow) more details
Why is Doxorubicin a “red” chemotherapy?
Colonies of microorganisms Streptomyces peucetius, which produce anthracyclines, have a characteristic red color. The medicine in the bottle is of a similar color. That's why women undergoing treatment for breast cancer call this chemotherapy "red."
“Red” chemotherapy is feared because it causes more severe side effects than “white,” “yellow,” and “blue” drugs. But there is no reason to be afraid: before prescribing a medicine, the doctor will thoroughly assess the patient’s health, weigh the pros and cons, prescribe supportive therapy and will constantly monitor the condition during the treatment process. Euroonko patients tolerate any chemotherapy comfortably - this is one of the most important principles of our work.
How is the patient's condition monitored during treatment?
Before prescribing Doxorubicin, general and biochemical blood tests are performed, the condition of the kidneys, liver, and heart is assessed. The tests are repeated during and after the course of chemotherapy. If you are bothered by any symptoms, you should immediately tell your doctor about them.
Sometimes Doxorubicin has to be stopped due to side effects, in which case the doctor will select another drug. At Euroonco, you can create a “molecular portrait” of a tumor and select the optimal combination of chemotherapy drugs, prescribing personalized chemotherapy that will be most effective for a particular patient.
Due to the pronounced side effects, many patients perceive Doxorubicin as a “powerful” chemotherapy drug. But resistance to it sometimes develops—the tumor becomes resistant and no longer responds to treatment. In such cases, other drugs are prescribed.
Pregnancy and Fertility
The drug is dangerous for the fetus, therefore it is impossible for a woman to become pregnant during treatment with Doxorubicin and for at least 6 months before and after completion of the course. You can have sex during chemotherapy, but you must use effective contraception. The drug passes into breast milk, so breastfeeding is also contraindicated.
After using Doxorubicin, problems with conceiving a child may occur. 10% of patients develop infertility. Therefore, if the patient plans to have children in the future, this issue should be discussed with the doctor in advance. A chemotherapy doctor may recommend sperm cryopreservation for a man and egg cryopreservation for a woman.
Book a consultation 24 hours a day
+7+7+78