Chemical properties
What is alpha lipoic acid ? Thioctic acid also has the names thioctacid , lipoic acid . It is a vitamin-like substance, a cofactor of pyruvate dehydrogenase and alpha-ketoglutarate dehydrogenase complexes, and an antioxidant .
The substance is synthesized in the form of a light yellow crystalline bitter powder, which is insoluble in water, but highly soluble in ethanol. In medicines, a soluble form of a chemical compound is used - its sodium salt . The substance is found in large quantities in liver, spinach, kidneys and heart, and rice. The body is normally able to synthesize sufficient amounts of alpha lipoic acid . The medicine is produced in the form of a concentrate for infusion solution and intramuscular injection, in the form of film-coated tablets.
Alpha lipoic acid in bodybuilding
The substance is used by athletes to eliminate free radicals and reduce oxidation levels after training. The product slows down the processes of destruction of proteins and cells, speeds up recovery after training. The substance also accelerates and improves the absorption of glucose by muscles and stimulates the processes of glycogen . It is also believed that the acid can be used as an effective fat burner.
Multiple sclerosis (MS) is a progressive autoimmune disease of the central nervous system (CNS) with a degenerative component, affecting mainly young patients and often leading to disability [1].
Recently, significant progress has been made in the world in the development and implementation of course-modifying drugs for multiple sclerosis (MDTs), which affect the reduction in the frequency of exacerbations of MS. DMTs registered in the Russian Federation [2] include interferons, glatiramer acetate, natalizumab, fingolimod and intravenous immunoglobulins; new drugs are being developed and prepared for registration. However, issues of symptomatic therapy and standards for stopping exacerbation (exacerbation) of the disease have not yet been completely resolved. The most effective methods of massive immunosuppression have been shown - pulse therapy with methylprednisolone, the use of exchange plasmapheresis, and to a lesser extent, large doses of glucocorticoids and oral immunosuppressants are prescribed [2]. At the same time, data are emerging on the possibility of influencing individual pathogenetic mechanisms to stop exacerbation of MS, in particular with the help of antioxidant therapy [3]. Moreover, various authors suggest using alternative approaches both along with international standards for the treatment of exacerbations, and instead of them (for example, with “mild” exacerbations or those accompanied only by sensitive symptoms). The great significance of this direction lies in the fact that with frequent use of hormone therapy, the effectiveness of repeated courses of immunosuppression is steadily decreasing, and the possibility of using alternative drugs to relieve exacerbations of moderate severity will allow maintaining the effectiveness of the standard pulse therapy regimen.
Ticotic (α-lipoic) acid (ALA) is an endogenous antioxidant, which is a coenzyme that is part of the cocarboxylase group of enzymes involved in carbohydrate and fat metabolism [4]. ALA, which has a pronounced antioxidant effect, has been shown in numerous studies to be effective in the treatment of diseases of the peripheral nervous system and central nervous system [5]. In the treatment of diabetic polyneuropathy, thioctic acid, based on a meta-analysis, is recognized as the only drug with class A level of evidence [6–9]. Many studies have been conducted on the effectiveness of thioctic acid in diseases of the central nervous system: multiple sclerosis [10, 11], acute cerebrovascular accident [12], traumatic brain injury [14], Alzheimer's disease [15], Parkinson's disease [16]. The drugs have been shown to be significantly effective in the treatment of experimental autoimmune encephalitis, the main model of demyelinating diseases in laboratory animals [9].
The degree of inhibition of inducible nitroxide synthase, matrix metalloprotease-9 (MMP-9), soluble intracellular adhesion molecules-1 (sICAM-1), nitric oxide, etc. are proposed as biochemical indicators of the effectiveness of antioxidant therapy [10, 11]. These indicators are not only related with oxidative stress, but also correlate with the activity of the process of migration of activated T-lymphocytes through the blood-brain barrier into the central nervous system. Several independent research groups have found a significant decrease in MMP-9, sICAM-1 with the use of both PITRS and thioctic acid [10, 11].
Taking into account the role of oxidative stress in the pathogenesis of demyelination and the maintenance of the autoimmune process in patients with MS, promising directions for optimizing the treatment of this disease are slowing down the processes of nitric oxide formation, introducing “scavenger” molecules from the outside, or enhancing enzymatic pathways of inactivation of free radicals. It is known that some of the well-known methods of treating MS patients have a pathogenetic effect on these processes. Thus, one of the effects of pulse therapy with methylprednisolone is a significant reduction in the formation of superoxide.
Currently, several drugs are produced containing various salts of thioctic acid (ethylenediamine, trometamol, megluminic). When administering the meglumine salt of thioctic acid, the incidence of side effects is lower than with the infusion of other thioctic acid salts.
A number of authors consider it necessary to further study the use of thioctic acid as a drug that reduces the likelihood of exacerbation, a means of symptomatic therapy, as well as the treatment of exacerbations in the form of monotherapy or as part of complex therapy.
The purpose of our work was to evaluate the effectiveness of the use of the drug α-lipoic acid in relieving exacerbation in patients with MS.
Material and methods
A cross-sectional prospective study was conducted involving patients from a neurological department with exacerbation of MS. The patients' condition was assessed using the Kurtzke EDSS (Expanded Disability Status Scale).
Criteria for inclusion in the study: age from 18 to 45 years, presence of exacerbation of MS, admission to the department within 10 days from the onset of exacerbation, presence of sensory disorders in the structure of the disease, informed voluntary consent to participate in the study. A total of 42 patients with exacerbation of MS were included in the study, randomly divided into two groups.
Group I – 22 patients with exacerbation of MS, with sensory impairments, aged from 19 to 36 years. On average 24.27±2.61 years. Among the examined there were 15 (68.18%) women and 7 (31.82%) men. Group II – 20 patients with exacerbation of MS, with sensory impairments, aged from 18 to 42 years.
On average 23.93±1.55 years. Among those examined there were 14 (70%) women and 6 (30%) men.
Treatment of all patients with exacerbation of MS was carried out taking into account clinical recommendations according to the regimen: methylprednisolone 1000 mg intravenously, by drip, for 5–7 days, depending on the severity of the exacerbation.
The ALA drug was added to the treatment of patients in group II according to the scheme: 600 mg (24 ml) of α-lipoic acid intravenously, drip, slowly in 200 ml of physiological solution daily for 5 days, then taking α-lipoic acid 600 mg 1 time per day orally within 5 weeks.
Clinical symptoms were assessed before the start of treatment, 2 and 6 weeks after the start of therapy using the Kurztke EDSS extended disability scale for MS.
Statistical processing of the results obtained and construction of diagrams was carried out using Microsoft Excel 5.0 for Windows and SPSS 15.0 for Windows.
Assessment of the effectiveness of the intervention is based on a clinically significant treatment outcome (absence or persistence of residual clinical symptoms of exacerbation of MS). Groups of patients were compared depending on the treatment regimen used, followed by the construction of a contingency table [17].
Research results
The figure shows the dynamics of clinical symptoms according to the EDSS scale in the study groups before the start of therapy, 2 weeks and 1 month after the start of treatment. The degree of disability on the EDSS scale at the time of the start of treatment for an exacerbation was higher in group II compared to group I (average value - 3.35 and 3.11 points, respectively). Despite the random assignment of patients to groups, the groups were absolutely comparable by gender and age and at the end of the study; the average EDSS was practically the same (2.07 points in group I and 2.0 in group II). However, there was a significantly better dynamics of symptom reduction as assessed by the total EDSS score in patients of group II who received ALA drugs along with pulse therapy with methylprednisolone. The dynamics during hospitalization (on average 14 days) for group I was -0.91 points on the EDSS scale, in group II the decrease was -1.02 points. One month after discharge (6 weeks from the start of therapy): overall dynamics according to EDSS for group I - -1.04 points, for group II - -1.35. When analyzing the dynamics of indicators of individual functional systems, statistically reliable patterns could not be identified due to the small sample of patients.
In patients of group I (22 people), the expected positive result (absence of new clinical symptoms in relation to the neurological status before the exacerbation) was observed in 6 (27.27%) people; probable unsatisfactory outcome (preservation of residual clinical symptoms) – in 16 (72.73%).
In patients of group II (20 people), the expected positive result (absence of residual clinical symptoms in relation to the neurological status before the exacerbation) was observed in 15 (75%) people; probable unsatisfactory outcome (preservation of residual clinical symptoms) – in 5 (25%).
NIL (frequency of outcomes in the experimental group)=A/(A+B)=5/20=0.25=25.0%.
CIC (rate of outcomes in the comparison group)=C/(C+D)=16/22=0.727≈72.7%.
SAR (absolute risk reduction)=/CHIL-CHIK/=/0.25-0.727/=0.477≈0.48≈48%.
RRR (relative risk reduction)=/CHIL-CHIK/CHIK≈ 0.477/0.727≈0.656≈65.6%.
NNT (the number of patients who need to be treated with a certain method for a given time in order to prevent the onset of an unfavorable outcome in one patient) = 1/SAP = 1/0.48≈1.92.
OR (odds ratio)=(A/B)/(C/D)=(5/15)/(16/6)=0.33/2.67≈0.12.
The obtained indicators indicate that the treatment regimen for exacerbation of MS is accompanied by a clinically significant positive effect, reflected in the ROR indicators of 65.6%, OR equal to ≈ 0.12. The NNT value, equal to 1.92 in our study, proves the possibility of preventing an unfavorable outcome (preservation of residual clinical symptoms) in at least every third patient with MS when using a treatment regimen for the disease using ALC.
Discussion
Currently, the role of α-lipoic acid preparations in the treatment of diseases of the nervous system, in the pathogenesis of which oxidative stress plays an active role, is increasingly being discussed. One of the most actively researched areas is the use of ALA in multiple sclerosis. Several studies have shown good tolerability of both oral and injectable forms of ALA preparations and their effectiveness as a means of symptomatic treatment of sensory disorders [10, 11]. The effect has been proven in terms of a significant reduction in serum markers of oxidative stress and a decrease in the permeability of the blood-brain barrier in the experiment [10].
Our small prospective study assessed the tolerability and effectiveness of ALA drugs for patients with exacerbation of MS and the possibility of improving prognosis. A more pronounced dynamics of decrease in EDSS was revealed in the group of patients who received thioctic acid drugs along with hormonal pulse therapy. Moreover, this dynamics was observed both in the acute period - 14 days from the start of treatment, and with continued use of thioctic acid drugs for 4 weeks after discharge from the hospital. The results of many studies give hope for the effective use of ALA drugs in the complex treatment of MS, but further larger studies are needed.
ALA preparations demonstrated good tolerability by all patients; not a single case of refusal of therapy due to side effects was recorded. From the standpoint of classical evidence-based medicine, the RRR of 65.6%, the OR level of 0.12 and NNT≈1.92 suggest a high degree of effectiveness of α-lipoic acid in the treatment regimen for exacerbation of MS. However, taking into account the small sample of patients and their heterogeneity in severity of exacerbation and duration of the disease, it is necessary to conduct large controlled studies in accordance with all the rules of evidence-based medicine to justify the need to prescribe the study drug as part of complex therapy for exacerbation of MS.
Pharmacodynamics and pharmacokinetics
Thioctic Acid is a coenzyme for the oxidative decarboxylation of pyruvic acid and various alpha-keto acids . cholesterol metabolism , and binds free radicals. Under the influence of the drug, liver function improves and glycogen . The effect of exogenous and endogenous toxins and alcohol is neutralized. In terms of its biochemical activity, the medicine is close to B vitamins .
When alpha-lipoic acid to solutions for intravenous administration (if the solutions are compatible), the severity of adverse drug reactions decreases.
After oral administration, preferably without food, the substance is completely and quickly absorbed from the digestive tract. Bioavailability reaches 30-60%, since the product undergoes presystemic biotransformation. The drug is oxidized in the liver tissue. Excreted via the kidneys. The half-life ranges from 20 minutes to an hour.
Lipoic acid 25 mg
Lipoic acid
Registration number and date: РN 001574/01 dated 05/10/2007
Trade name of the drug: Lipoic acid
International nonproprietary name: thioctic acid Dosage form: film-coated tablets
Compound:
Active substance:
Lipoic acid (thioctic acid) -12 mg and 25 mg
Excipients: sugar (sucrose), glucose (dextrose), potato starch, calcium stearate 1-water (calcium stearate monohydrate), stearic acid, talc (magnesium hydrosilicate).
Excipients of the shell: aerosil (colloidal silicon dioxide), beeswax, titanium dioxide, basic magnesium carbonate (magnesium hydroxycarbonate), vaseline oil, low molecular weight medical polyvinylpyrrolidone (povidone), sugar (sucrose), talc (magnesium hydrosilicate), quinoline yellow dye E -104.
Description
Yellow or greenish-yellow film-coated tablets.
The cross section shows two layers.
Pharmacotherapeutic group: metabolic agent.
ATX code : [A05BA].
pharmachologic effect
Lipoic acid is a coenzyme involved in the oxidative decarboxylation of pyruvic acid and alpha-keto acids and plays an important role in the body's energy balance. By the nature of its biochemical action, thioctic acid is similar to B vitamins. It participates in the regulation of lipid and carbohydrate metabolism, has a lipotropic effect, affects cholesterol metabolism, improves liver function, and has a detoxification effect in case of poisoning with heavy metal salts and other intoxications.
Indications for use
Fatty liver, liver cirrhosis, chronic hepatitis, hepatitis A, intoxication (including salts of heavy metals, toadstool), hyperlipidemia.
Contraindications
Hypersensitivity, lactation period, children's age (up to 6 years).
With caution - pregnancy
Directions for use and doses
Inside after meals. Adults - 50 mg 3-4 times a day.
Children over 6 years old - 12-24 mg 2-3 times a day.
The duration of treatment is 20-30 days. If necessary, as prescribed by a doctor, a second course of treatment is carried out after 1 month.
Side effect
Dyspepsia (including nausea, heartburn, vomiting, diarrhea, abdominal pain), allergic reactions (including urticaria, skin rash, itching and systemic allergic reactions up to anaphylactic shock), hypoglycemia.
Interaction with other drugs
Strengthens the anti-inflammatory effect of glucocorticosteroids. Reduces the effectiveness of cisplatin. Enhances the effect of insulin and oral hypoglycemic agents. Binds metals, so should not be taken simultaneously with drugs containing metal ions (iron, calcium magnesium); the interval between doses should be at least 2 hours. Ethanol and its metabolites weaken the effect of lipoic acid.
special instructions
During the treatment period, regular monitoring of glucose concentrations (especially at the beginning of therapy) in patients with diabetes mellitus is necessary; you should refrain from drinking alcohol.
Release form
Film-coated tablets 12 mg and 25 mg.
10 tablets per blister pack.
50, 100 tablets in glass jars or polymer jars.
Each jar or 5 blister packs along with instructions for use are placed in a pack.
Conditions for dispensing from pharmacies
On prescription.
Storage conditions:
In a dry place, protected from light, at a temperature not exceeding 25°C.
Best before date:
3 years. Do not use after the expiration date stated on the package.
Units:
pack
Side effects
The following side effects may develop:
- vomiting, diarrhea , nausea, abdominal pain, urticaria ;
- itching and rash , anaphylactic reactions , hypoglycemia ;
- headaches , hypoglycemia ;
- after rapid intravenous administration - breath holding, increased intracranial pressure , diplopia , convulsions , bleeding.
Thioctic acid, instructions for use (Method and dosage)
When prescribing the drug orally, it is used in a single dosage of 600 mg. The course of treatment is long, on average 3 months.
Instructions for alpha lipoic acid for injection
For severe polyneuropathy , 600 mg of the drug is administered intravenously, slowly, 50 mg per minute. The concentrate is diluted with sodium chloride . The frequency of administration is once a day. If necessary, the dose can be increased to 1.2 g per day. Duration of treatment is up to 4 weeks.
It is not recommended to administer more than 50 mg of the drug intramuscularly at a time. It is necessary to periodically change the injection site.
Alpha lipoic Evalar is taken according to the manufacturer's instructions.
Efficacy of thioctic acid preparations in the treatment of diabetic polyneuropathy
M.V. NESTEROVA1
, Doctor of Medical Sciences,
V.V.
GALKIN2 1
Ural State Medical University, Sverdlovsk Regional Clinical Psychoneurological Hospital for War Veterans, Yekaterinburg
2
Demidov City Hospital, Nizhny Tagil The article discusses the treatment of diabetic polyneuropathy - the most common form of neurological complication of diabetes mellitus.
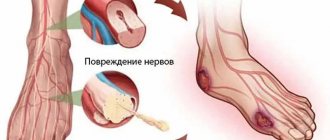
The pathogenesis, features of diagnosis and treatment from the modern perspective of evidence-based medicine are presented. An analysis of thioctic acid preparations existing on the Russian pharmaceutical market for the treatment of diabetic polyneuropathy was carried out, indicating the most preferred trade names for use. The results of our own research on the treatment of painful diabetic neuropathy are presented. The problem of treating diabetic polyneuropathy (DPN), one of the most common forms of neurological complications of diabetes mellitus (DM), continues to remain relevant due to the widespread and significant material losses caused to society as a result of damage associated with disability. DPN, along with diabetic retinopathy and nephropathy, is part of the classic triad of late complications of diabetes and leads to a deterioration in the quality of life, disability and death of patients. With the introduction of new insulin drugs into practice, the life expectancy of patients with diabetes mellitus has increased significantly, and therefore the percentage of late complications, including DPN, has increased. DPN is an interdisciplinary subject of attention and is found in the practice of not only neurologists and endocrinologists, but also therapists, dermatologists, pediatric surgeons, rehabilitation specialists and other specialists.
Diabetes mellitus is one of the most common diseases, affecting up to 10-15% of the population of developed countries with the number of patients doubling every 10-15 years [1]. In the literature, according to various researchers, DPN develops at different times in almost all patients with diabetes; the frequency varies widely and is detected in almost 50% of patients with diabetes and 30% of all cases of polyneuropathy [2–11]. According to the Rochester study, the prevalence of DPN in North America is about 50%, which corresponds to the prevalence of DPN in the European population of patients with diabetes [5, 12-14]. A number of authors indicate the likelihood of developing neuropathy depending on the compensation of carbohydrate metabolism, duration of diabetes, type of diabetes and is diagnosed in 7.5-10% of patients with newly diagnosed type 2 diabetes [7-9]. Other authors note the same prevalence of DPN in type 1 and type 2 diabetes and the dependence on the duration of diabetes and the effectiveness of its treatment [15-19]. The frequency of its detection directly depends on the diagnostic criteria used in various population studies. Some researchers indicate that the frequency of diagnosed neuropathy during electroneuromyography is up to 90-100%. Up to 50% of patients are asymptomatic, and pain syndrome in DPN occurs in approximately 10-20% of cases and is one of the most painful manifestations of the disease for the patient [3, 20, 21]. In 2006, M. Davies et al. published data from a study in which up to 64% of patients complained of pain, while only 19% had “true” painful DPN [22]. There was a clear correlation between the presence and severity of painful neuropathy with the duration of diabetes, the level of glycosylated hemoglobin (HbA1c) and the severity of neuropathy. It is now recognized that the incidence of DPN is associated with the duration of diabetes, the level and significant fluctuations of HbA1c, dyslipidemia, high body mass index, albuminuria, hypertension and smoking [18, 19, 23].
At the present stage of development of diabetology and neurology, there are a large number of different theories regarding the causes and mechanisms of development of DPN, among which researchers identify four main ones: metabolic, vascular, hereditary and dysimmune theories [2, 24-27]. Despite the abundance of concepts for the development of neuropathy, its main established pathogenetic factor is hyperglycemia [28, 29]. Pfieffer and Schumer suggested that the temporary pathogenesis of DPN can be divided into a stage of functional and a stage of anatomical disorders [30]. Functional changes develop in the early stages of neuropathy and are fully or partially reversible against the background of stable glycemic control, which is confirmed by the DCCT and UKPDS studies, in which patients with long-term compensated diabetes were able to reduce the development of DPN (by 64%) compared with patients with decompensated diabetes. DM [10, 31]. Hyperglycemia causes neuronal damage caused by an intracellular increase in glucose with excess metabolism and accumulation of toxic products such as sorbitol and fructose, increased intracellular osmotic pressure, and neuronal edema. It is noted that hyperglycemia with the accumulation of toxic metabolites and advanced glycation end products increases the formation of free radicals with the development of “oxidative stress” and a decrease in the level of myoinositol, which also has a damaging effect on neurons. Currently, the phenomenon of “oxidative stress” is considered as the main cause of late complications of diabetes, including generalized damage to peripheral nerves. A decrease in nerve growth factors in DPN impairs the regenerative capabilities of axons, which contributes to the progression of axonal degeneration and segmental demyelination. According to numerous large controlled studies, such as DCCT, UKPDS, Rochester cohort, VACSDM, Steno-2, a clear connection has been established between the duration of diabetes and the degree of hyperglycemia with the severity of polyneuropathy [5, 10, 31-35]. The vascular theory, equally with the metabolic one, is also considered one of the leading ones in the pathogenesis of DPN. Academician A.S. Efimov wrote in his works: “...diabetes, starting as a metabolic disease, becomes predominantly a vascular pathology” [36, 37]. Attention is paid to microangiopathy, which also develops as a result of glycation of endothelial cells, in which the vasa nervorum is affected and the endoneurial circulation is disrupted, with the formation of pericapillary edema. The vascular theory also explains the development of diabetic retinopathy and nephropathy [28, 29]. Endoneural hypoxia, metabolic changes and impaired production of vasoactive relaxants (nitric oxide) contribute to the development of nerve ischemia, resulting in degenerative and apoptotic changes in peripheral nerves.
According to the WHO definition, DPN is a disease characterized by the progressive death of nerve fibers, leading to loss of sensation and the development of foot ulcers [38, 39]. Due to the variability of clinical manifestations, there is no universal classification of DPN in the literature. The most widely used classification is PK Thomas (1997, 2003), according to which the following clinical forms are distinguished: reversible (transient hyperglycemic neuropathy, acute sensory painful neuropathy, cranial, multiple mononeuropathies, radiculoplexopathies) and progressive (distal sensorimotor polyneuropathy, proximal motor neuropathy, autonomic neuropathy I ) [40, 41]. A.S. Efimov et al. in 1981, they proposed a classification considering preclinical, initial, overt and severe stages of DPN [37]. This classification is close to the division of neuropathy into four stages proposed by PJ Dyck et al. (1993, 1999, 2003): asymptomatic (N1a), mild (N1b and N2a), moderate (N2b) and severe disabling (N3) stages of DPN [37, 42].
Currently, sufficient factual material on the treatment of diabetic neuropathy has been accumulated in the literature. The main directions in treatment are the impact on pathogenesis and the prescription of symptomatic remedies.
Pathogenetic treatment of diabetic neuropathy includes compensation of carbohydrate metabolism with the administration of neurometabolic agents. Normalizing blood sugar levels significantly reduces the risk of developing polyneuropathy. The DCCT (Diabetes Control and Complications Trial) study showed that normalization of carbohydrate metabolism and adequate glycemic control subsequently reduce the risk of developing DPN by 64% and the incidence of nerve conduction disorders in patients with type 1 diabetes by 44%. It was also noted that, against the background of stable glycemic control, patients with newly diagnosed DPN showed regression of the clinical manifestations of this complication [31]. Similar conclusions were obtained in the UKPDS study conducted in the UK, where it was shown that patients with type 2 diabetes who received intensive glucose-lowering therapy with a decrease in HbA1c levels to 7.0% had a significant reduction of 40% in the incidence of polyneuropathy according to compared with patients on standard therapy [10]. But it turned out that intensive hypoglycemic therapy is not able to completely eliminate the occurrence or eliminate the clinical manifestations of DPN and lead to its serious regression. In this regard, additional pathogenetic and symptomatic treatment is required.
Over the years, many neurometabolic agents have been proposed for the treatment of diabetic neuropathy. The main drugs that have a pathogenetic effect are antioxidants and fat-soluble thiamine derivatives.
Antioxidant drugs reduce the severity of oxidative stress by acting as free radical scavengers. The bulk of data on the effectiveness of antioxidants in DPN in the literature is presented with the use of α-lipoic (thioctic) acid [43-45]. The therapeutic effect of α-lipoic (thioctic) acid lies in its powerful antioxidant effect. Under the influence of α-lipoic (thioctic) acid, the synthesis of cyclic adenosine monophosphate occurs through the activation of receptors associated with G-protein and adenylate cyclase, as well as the regeneration and restoration of other antioxidants in the body through the effect on tissue glutathione and ubiquinone [46]. α-lipoic (thioctic) acid can also act as a coenzyme of multienzyme complexes for the oxidative decarboxylation of pyruvic and alpha-keto acids. α-Lipoic (thioctic) acid can prevent the formation of advanced glycation end products, reducing the severity of oxidative stress [47]. α-Lipoic (thioctic) acid is a universal free radical scavenger that works both inside and outside the cell. The first use of α-lipoic (thioctic) acid in the clinic for the treatment of DPN was carried out back in 1959. Further preclinical and clinical studies showed that it affects the pathogenesis, risk factors for the onset and progression of DPN [48]. The high effectiveness of α-lipoic (thioctic) acid has been confirmed in several randomized, double-blind, placebo-controlled studies, such as ALADIN, ALADIN II, ALADIN III, ORPIL, SYDNEY, DEKAN, NATHAN I and NATHAN II [49-54]. Controlled studies have shown that the use of α-lipoic (thioctic) acid drugs reduces both subjective (pain, burning, numbness, paresthesia) and objective manifestations of diabetic polyneuropathy, cardiac autonomic DN, in particular increases heart rate variability compared to placebo [ 55]. Long-term oral therapy with α-lipoic (thioctic) acid has been shown to not only control the symptoms of peripheral DN, but also improve electrophysiological parameters of nerve function [52]. Doses of 600 and 1,200 mg per day were equally effective, but the 1,200 mg dose was associated with a higher incidence of side effects. In addition, treatment with α-lipoic (thioctic) acid has been shown to be well tolerated and safe. A dose of α-lipoic (thioctic) acid of 600 mg is the most optimal in terms of benefit-risk [56]. In 2010, S. Salinthone et al. showed anti-inflammatory effects of α-lipoic (thioctic) acid. Thus, α-lipoic (thioctic) acid suppresses the activity and cytotoxicity of NK cells, reduces the level of interleukins 6 and 17, and the proliferation of T cells [46]. Two randomized, placebo-controlled studies demonstrated the ability of α-lipoic (thioctic) acid to increase tissue glucose utilization and improve the glycemic profile in diabetic patients treated with α-lipoic (thioctic) acid [57–59]. Recently, α-lipoic (thioctic) acid has been used in the treatment of other complications of diabetes, such as nephropathy and retinopathy [60–63].
In a large clinical trial ESPALIPON II (1995) involving 3,509 patients with DPN, high efficacy and good tolerability of the thioctic acid drug Espa-Lipon in various dosage forms were demonstrated. This applied to both the solution for infusion and the tablet form of the drug. Effectiveness was assessed as “very good” or “good” by 83% of doctors and 80% of patients, which was confirmed by clinical indicators. The tolerability of the drug Espa-Lipon was also highly rated - 95% of doctors and patients spoke in favor of “good” and “very good” tolerability of the drug [68].
Preparations of α-lipoic (thioctic) acid are substances with poor solubility; therefore, tablet preparations of α-lipoic (thioctic) acid differ in the degree of release of the active substance ( Fig.
.). The difference in bioavailability and high variability in plasma concentrations of oral preparations of α-lipoic (thioctic) acid do not allow us to talk about their interchangeability [48]. Randomized controlled clinical trials of α-lipoic (thioctic) acid were conducted using a “rapid release” formulation that has a reference solubility test and the lowest interindividual variability in plasma levels. Espa-Lipon is as close as possible to a “quick release” drug.
The modern drug market offers more than 14 trade names of drugs with the active ingredient lipoic (thioctic) acid, available in various forms. α-Lipoic (thioctic) acid preparations are produced by both domestic and foreign manufacturers. Our country produces four types of medicines. Drugs from foreign companies are represented by five names and are produced mainly by Germany and Ukraine. In the table
The main drugs for α-lipoic (thioctic) acid are presented.
| Table. Basic drugs α-lipoic (thioctic) acid | ||||
| Trade name | Firm | A country | Release form | Pack, pcs. |
| Tablet and capsule forms | ||||
| Berlition 300 | "Berlin-Pharma" | Germany | Table po 300 mg | 30 |
| Neurolipon | "Farmak PJSC" | Ukraine | Caps. 300 mg | 30, 60 |
| Octolipen | "Pharmstandard-Leksredstva" | Russia | Caps. 300 mg, tab. po 600 mg | 30 |
| Thiogamma | Verwag Pharma GmbH & Co. Kg" | Germany | Table po 600 mg | 30, 60 |
| Thioctacid BV | Meda Pharma GmbH & Co. Kg" | Germany | Table po 600 mg | 30, 60, 100 |
| Thiolepta | "Canonpharma Production" | Russia | Table p/o 300, 600 mg | 30 |
| Espa-Lipon | Esparma GmbH | Germany | Table po 600 mg | 30 |
| Infusion forms | ||||
| Berlition 300 Berlition 600 | "Berlin-Pharma" | Germany | Amp. 25 mg/ml, 12, 24 ml | 5 |
| Lipothioxone | Farm | Russia | Amp. 25 mg/ml, 12, 24 ml | 5 |
| Neurolipon | "Farmak PJSC" | Ukraine | Amp. 30 mg/ml, 10, 20 ml | 5 |
| Octolipen | "Pharmstandard-UfaVITA" | Russia | Amp. 30 mg/ml, 5, 10 ml | 5, 10 |
| Thiogamma | Verwag Pharma GmbH & Co. Kg" | Germany | Amp. 12 mg/ml, 50 ml, 30 mg/ml, 20 ml | 5, 10 |
| Thioctacid 600 T | Meda Pharma GmbH & Co. Kg" | Germany | Amp. 25 mg/ml, 24 ml | 5 |
| Thiolipon | "Biosynthesis" | Russia | Amp. 30 mg/ml, 10 ml | 10 |
| Espa-Lipon | Esparma GmbH | Germany | Amp. 25 mg/ml, 12 ml | 10 |
| Espa-Lipon | Esparma GmbH | Germany | Amp. 25 mg/ml, 24 ml | 5 |
It should be noted that treatment with Espa-Lipon in a daily dosage of 600 mg has advantages in terms of pharmacoeconomic indicators compared to therapy with other α-lipoic acid preparations from German manufacturers. As for quality, the drug Espa-Lipon is far superior to its Russian and Ukrainian counterparts.
Infusion preparations of α-lipoic (thioctic) acid are represented by three salts: ethylenediamine (Espa-Lipon, Berlition, Octolipen), trometamol (Thioctacid) and megluminic (Tiogamma, Neurolipon). It is believed that meglumine and trometomol salts, when administered intravenously, are more likely than ethylenediamine salt to cause a local irritant effect on tissue and a burning sensation, a decrease in blood pressure and headache. In Russia, the drug Espa-Lipon (Esparma GmbH) has been used for more than 15 years. The drug is available in the form of a concentrate of ethylenediamine salt of α-lipoic (thioctic) acid for the preparation of a solution in the form of infusions of 300 or 600 mg and tablets of 600 mg and is dispensed according to prescribed prescriptions. In clinical studies, the drug Espa-Lipon showed its high effectiveness [64]. During treatment, an improvement in the symptoms of diabetic neuropathy was observed. In 95% of cases, treating physicians and 95% of patients rated the drug’s tolerability as “good” or “very good.” Side effects included mild nausea, headaches, dizziness, dyspepsia, as well as a burning sensation and itching after rapid administration of the drug. Espa-Lipon dosage regimen: for severe forms of polyneuropathy, the drug is prescribed once a day (in the morning on an empty stomach 30-40 minutes before meals) in the form of intravenous drip infusions. For adults, to prepare an infusion solution, 24 to 48 ml of solution is diluted in 250 ml of isotonic sodium chloride solution (which corresponds to taking 600-1,200 mg of thioctic acid per day), depending on the severity of the condition and the patient’s body weight. The drug is recommended to be used for 2-4 weeks. IM administration of the drug is possible, but the dose of the drug when injected into the same place should not exceed 50 mg (2 ml).
Next, you should switch to maintenance therapy in the form of tablets. The minimum course of taking tablets is 3 months. The average recommended dose is 400-600 mg/day (1 tablet of 600 mg or 2-3 tablets of 200 mg). If necessary, longer use of the drug is possible.
The tablets should be taken 30 minutes before meals, without chewing and with a small amount of liquid.
In DPN, it is important to determine indications for symptomatic therapy. For this purpose, a number of authors recommend the use of a visual analogue scale (VAS) (or Lickert scale). Symptomatic therapy is prescribed for VAS scores > 40 mm, as well as for sleep disturbance due to pain and decreased quality of life [65–67]. Neuropathic pain, unlike nociceptive pain, is difficult to treat with conventional analgesics and non-steroidal anti-inflammatory drugs, and their use - in patients with diabetic nephropathy - can be dangerous. Pain in polyneuropathy can be controlled using several groups of neurotropic drugs that act on different mechanisms of neuropathic pain. These include antidepressants, anticonvulsants, local anesthetics and opioid analgesics.
The authors, at the Demidov City Hospital and the Sverdlovsk Regional Clinical Psychoneurological Hospital, conducted a study on the treatment of diabetic neuropathy with symptomatic drugs. For this purpose, the drugs duloxetine and gabapentin were used, which were prescribed for the painful form of diabetic neuropathy. Both drugs significantly reduce all manifestations of neuropathic pain in diabetic neuropathy, but have differences in the severity of the analgesic effect and in biological age in favor of duloxetine, as well as differences in the treatment of objective symptoms. Thus, gabapentin restored tendon reflexes and vibration sensitivity, and duloxetine improved pain, temperature and tactile sensitivity.
Physiotherapeutic techniques are used to treat DPN, such as magnetic therapy, transcutaneous electrical nerve stimulation, therapeutic exercises, balneotherapy, relaxation therapy, and acupuncture. The goal of physiotherapy for DPN is to provide analgesic, anti-inflammatory effects, improve the conduction of impulses along the nerves, accelerate the processes of regeneration of nerve fibers, and improve blood circulation in the perineural tissues.
Thus, diabetes, with its late complications such as diabetic neuropathy and diabetic foot syndrome, is a global epidemic due to its high prevalence, with significant human, social and economic consequences.
This makes the problem of searching and developing new therapeutic techniques urgent. Long-term therapy with α-lipoic (thioctic) acid (Espa-Lipon) reduces neurological deficits and reduces the risk of developing diabetic foot syndrome and limb amputation. The results collected to date allow us to consider the antioxidant Espa-Lipon as an extremely promising drug that has a protective effect on the mechanisms of development of neuropathy and other complications of diabetes. Literature
1. Dedov I.I. Diabetes mellitus in the Russian Federation: problems and solutions. Diabetes Mellitus, 1998, 1: 7-18. 2. Zhulev N.M. Neuropathies: A Guide for Doctors. St. Petersburg: Publishing House SPbMAPO, 2005, 416. 3. Yakhno N.N., Kukushkin M.L., Davydov O.S. and others. Results of the Russian epidemiological study of the prevalence of neuropathic pain, its causes and characteristics in the population of outpatients who consulted a neurologist (EPIC Study). Pain, 2008, 3: 24-32. 4. Cabezas-Cerrato J. The prevalence of diabetic neuropathy in Spain: a study in primary care and hospital clinic groups. Diabetologia, 1998, 41: 1263-1269. 5. Dyck PJ, Katz KM, Karnes JL et al. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathy Study. Neurology. 1993, 43: 817-824. 6. Kumar S, Ashe HC, Parnell LN et al. The prevalence of foot ulceration and its correlates in type 2 diabetes: a population-based study. Diabe Med 1994, 11: 480-484. 7. Melton LJ, Dyck PJ, Thomas PK. In Diabetic Neuropathy. Epidemiology, 1999: 239-278. 8. Partanen J, Niskanen L, Lehtinen J, Mervaala E et al. Natural history of peripheral neuropathy in patients with non-insulin dependent diabetes. New Engl J Med 1995, 333: 39-84. 9. Pirart J. Diabetes mellitus and its degenerative complications: a prospective study of 4,400 patients observed between 1947 and 1973. Diabetes Care. 1978, 1: 168-188. 10. UKPDS: Intensive blood glucose with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. Lancet. 1998, 352: 837–853. 11. Young MJ, Boulton AJM, MacLeod AF et al. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia. 1993, 36: 150–154. 12. Yakhno N.N. Pain. Guide for doctors and students. M., 2009. 304 p. 13. Bouhassira D, Lanteri-Minet M, Attal N et al. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. 2008, 136: 380–387. 14. Torrance N, Smith BH, Bennett MI et al. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. Pain. 2006, 7: 281-289. 15. Kalinin A.P., Kotov S.V., Rudakova I.G. Neurological disorders in endocrine diseases: A guide for doctors. M.: MIA, 2009, 488 p. 16. Franklin GM, Kahn LB, Baxter J et al. Sensory neuropathy in noninsulin-dependent diabetes mellitus. Am J Epidemiol. 1990: 633–643. 17. Prevalence of Diabetic Neuropathy and Foot Ulceration: Identification of Potential Risk Factors - A Population-Based Study. Manes Ch, Papazoglou N, Sossidou E et al. 14, 2002, Wounds, Vol. 1: 11-15. 18. Epidemiological correlates of diabetic neuropathy: report from Pittsburgh Epidemiology of Diabetes Complications Study. Maser RE, Steenkiste AR, Dorman JS, Nielsen VK et al. 38, 1989, Diabetes: 1456–1461. 19. Tesfaye S, Chaturvedi N, Simon EM et al. Vascular Risk Factors and Diabetic Neuropathy. The New England Journal of Medicine. 2005, 4, 352: 341-350. 20. Sorensen L, Molyneaux L, Yue DK. Insensate versus painful diabetic neuropathy: the effects of height, gender, ethnicity and glycemic control. Diabetes Res Clin Pract. 2002, 57: 45-51. 21. Ziegler D, Gries FA, Spuler M, Lessmann F. The epidemiology of diabetic neuropathy: DiaCAN Multicenter Study Group. Diabet Med. 1993, 10: 82-86. 22. Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care. 2006, 29: 1518-1522. 23. Franklin GM, Shetterly SM, Cohen JA, Baxter J, Hamman RF Risk factors for distal symmetric neuropathy in NIDDM. Diabetes Care. 1994, 17: 1172-1177. 24. Levin O.S. Polyneuropathy. M.: MIA, 2011. 496 p. 25. Pittenger GL, Malik RA, Burcus N, Boulton AJ, Vinik AI Specific fiber deficits in sensorimotor diabetic polyneuropathy correspond to cytotoxicity against neuroblastoma cells of sera from patients with diabetes. Diabetes Care. 1999, 22: 18. 26. Srinivasan S, Stevens MJ, Sheng H, Hall KE, Wiley JW. Serum from patients with type 2 diabetes with neuropathy induces complement-independent, calcium-dependent apoptosis in cultured neuronal cells. J Clin Invest. 1998, 102: 1454-1462. 27. Strokov IA, Bursa TR, Drepa OI et al. Predisposing genetic factors for diabetic polyneuropathy in patients with type 1 diabetes: a population—based case—control study. Acta diabetologica. 2003, 2, 40: 375-379. 28. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001, 414: 813-820. 29. Brownlee M. The pathobiology of diabetic complications. A unifying mechanism. Diabetes. 2005, 54: 1615-1625. 30. Pfiefer MA, Schumer MP. Clinical trials of diabetic neuropathy: Past, present and future. Diabetes. 1995, 44: 1355-1361. 31. DCCT (The Diabetes Control and Complications Trial Research Group). The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993, 329: 977-986. 32. Azad N, Emanuele NV, Abraira C, Henderson WG et al. The effects of intensive glycemic control on neuropathy in the VA Cooperative Study on Type II Diabetes Mellitus (VACSDM). J Diabetes Compl. 1999, 13: 307-313. 33. Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008, 358: 580-591. 34. Gaede P, Vedel P, Larsen N, Jensen GV et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003, 348: 383–393. 35. Pitale SU, Abraira C, Emanuele NV et al. Two years of intensive glycemic control and left ventricular function in the Veterans Affairs Cooperative Study in Type 2 Diabetes Mellitus (VACSDM). Diabetes Care. 2000, 23: 1316-1320. 36. Efimov A., Zueva N., Skrobonskaya N. Diabetic angiopathy: etiology and pathogenesis. Faces of Ukraine. 2004, 11: 36-38. 37. Efimov A.S. Diabetic angiopathy. M., 1989. 38. Dedov I.I., Antsiferov M.B., Galstyan G.R., Tokmakova A.Yu. Diabetic foot syndrome: clinical picture, diagnosis, treatment and prevention. M., 1998: 138 p. 39. Carrington AL, Abbott CA, Shaw JE, Vileikyte L, Van Schie CHM, Boulton AJM. Can motor nerve conduction velocity predict foot problems in diabetic neuropathy over a 6-year outcome period? Diabetes Care. 2002, 25: 2010-2015. 40. Thomas PK. Classification of the diabetic neuropathies. [author books] Cameron NE, Low PA, Ziegler D. Gries FA. Thomas PK.: Class Diabetic Neuropathy. Stuttgart: Thieme, 2003: 175-177. 41. Thomas PK. Classification, differential diagnosis and staging of diabetic peripheral neuropathy. Diabetes. 1997, 46: 54-57. 42. Dyck PJ. Severity and staging of diabetic polyneuropathy. [author books] Cameron NE, Low PA, Ziegler D. Gries FA. Diabetic Neuropathy. Stuttgart: Thieme, 2003: 170-175. 43. Severina T.I., Tarasov A.V., Trelskaya N.Yu., Shilova O.L., Drometr A.V. Results of the use of thioctacid in the treatment of diabetic neuropathy in patients with type 1 diabetes. Diabetes. 2000, 4: 33-35. 44. Strokov I.A., Strokov K.I., Akhmedzhanova L.L., Albekova Zh.S. Thioctacid in the treatment of diabetic polyneuropathy. Difficult patient. 2008, 12: 19-23. 45. Strokov I.A., Kozlova N.A., Mozolevsky Yu.V. and others. The effectiveness of intravenous administration of trometamol salt of thioctic (α-lipoic) acid in diabetic neuropathy. Journal of Neurology and Psychiatry. 1999, 99, 6: 18-22. 46. Salinthone S, Yadav V, Schillace RV et al. Lipoic acid attenuates inflammation via cAMP and protein kinase A signaling. PLoS One. 2010, 9, 5: 13058. 47. Menshchikova E.B., Lankin V.Z., Zenkov N.K. etc. Oxidative stress. Prooxidants and antioxidants. M.: Slovo, 2006: 553. 48. Belousov Yu.B., Afanasyeva E.V., Belousov D.Yu., Zyryanov S.K. The use of thioctic acid in the treatment of diabetic polyneuropathy. Quality clinical practice. 2011, 1: 85-91. 49. Ametov AS, Barinov A, Dyck PJ et al. The Sensory Symptoms of Diabetic Polyneuropathy Are Improved With Lipoic Acid (The SYDNEY Trial). Diabetes Care. 2003, 26: 770-776. 50. Reljanovic M, Reichel G, Rett K et al. Treatment of diabetic polyneuropathy with the antioxidant thioctic acid (alpha-lipoic acid): a two year multicentre randomized double-blind placebo-controlled trial (ALADIN II). Free Radic Res. 1999, 31: 17. 51. Ziegler D, Hanefeld M, Ruhnau K et al. And the ALADIN Study group: treatment of symptomatic diabetic peripheral neuropathy with the antioxidant a-lipoid acid. Diabetologica. 1995, 38: 1425-1433. 52. Ziegler D, Hanefeld M, Ruhnau KJ et al. Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: a 7-month multicentre randomized controlled trial (ALADIN III Study). ALADIN III Study Group. Diabetes Care. 1999, 22: 1296-1301. 53. Ziegler D, Nowak H, Kemplert P et al. Treatment of symptomatic diabetic polyneuropathy with antioxidant a-lipoic acid: a meta-analysis. Diabetic Medicine 2004. Vol. 21. P. 114-21. Diabetic Medicine. 2004, 21: 114-121. 54. Ziegler D, Ametov A, Barinov A et al. Oral treatment with alpha-lipoic acid improves symptomatic diabetic polyneuropathy: the SYDNEY 2 trial. Diabetes Care. 2006, 29: 2365-2370. 55. Ziegler D, Gries FA. Alpha-lipoic acid in the treatment of diabetic peripheral and cardiac autonomic neuropathy. Diabetes. 1997, Vol. 2, 46: 562-566. 56. Han T, Bai J, Liu W, Hu Y. A systematic review and meta-analysis of α-lipoic acid in the treatment of diabetic peripheral neuropathy. Eur. J. Endocrinol. 2012, 167: 465-471. 57. Menshchikova E.G., Zenkov N.K., Lankin V.Z. etc. Oxidative stress. Pathological conditions and diseases. Novosibirsk: ARTA, 2008. 284. 58. Ansar H, Mazloom Z, Kazemi F, Hejazi N. Effect of alpha-lipoic acid on blood glucose, insulin resistance and gluta-thione peroxidase of type 2 diabetic patients. Saudi. Med. J. 2011. 32. 6: 584–588. Saudi. Med. J. 2011, 6, 32: 584-588. 59. Porasuphatana S, Suddee S, Nartnampong A et al. Glycemic and oxidative status of patients with type 2 diabetes mellitus following oral administration of alpha-lipoic acid: a randomized double-blinded placebo-controlled study. Asia Pac. J. Clin. Nutr. 2012, 1, 21: 12-21. 60. Wang L, Wu CG, Fang CQ et al. The protective effect of α-Lipoic acid on mitochondria in the kidney of diabetic rats. Int. J. Clin. Exp. Med. 2013, 2, 6: 90-97. 61. Trakhenberg Yu.A., Milenkaya T.M., Ametov A.S., Demidova T.Yu. Studying the effectiveness of alpha-lipoic acid in patients with type 2 diabetes mellitus and non-proliferative diabetic retinopathy. Diabetes. 2006, 3: 39–41. 62. Heinisch BB, Francesconi M, Mittermayer F et al. Alpha-lipoic acid improves vascular endothelial function in patients with type 2 diabetes: a placebo-controlled randomized trial. Eur. J. Clin. Invest. 2010, 2, 40: 148-154. 63. Haritoglou C, Gerss J, Hammes HP et al. Alpha-lipoic acid for the prevention of diabetic macular edema. Ophthalmologica. 2011, 3, 226: 127-137. 64. Nedosugova L.V. Alpha lipoic acid (Espa-Lipon) in the complex treatment of diabetic neuropathy. International Journal of Endocrinology. 2007, 8, 2: 49-51. 65. Belova A.N. Neurorehabilitation. M., 2003, 734. 66. Bregovsky V.B. Painful forms of diabetic polyneuropathy of the lower extremities: modern concepts and treatment options (literature review). Pain. 2008, 1: 29-34. 67. Strokov I.A., Barinov A.N. Clinic, pathogenesis and treatment of pain syndrome in diabetic polyneuropathy. Neurological Journal. 2001, 6: 47-54. 68. Clinical study Espalipon II. Dosage 600 mg (study no. 616-14-94-002 05/02/1995). Source:
Medical Council, No. 5, 2015
Interaction
The medicine reduces the effectiveness of cisplatin and enhances the effect of oral hypoglycemic drugs and insulin .
The substance cannot be mixed in the same container with dextrose , Ringer's solution , ethanol, and solutions that react with SH groups and disulfide bridges.
The product enhances the effect of taking carnitine .
Ethanol and medications containing ethyl alcohol weaken the effect of taking acid.
Preparations containing (Thioctic Acid Analogues)
Level 4 ATX code matches:
Verona
Phibs
Gastrikumel
Fitogastrol
Thyroidea Compositum
Berlition
Thiolepta
Gastric collection
Espa-Lipon
Brewer's yeast
Lipoic acid
Rosehip syrup
Octolipen
Thioctacid
Thiogamma
Figurin
There are a lot of drugs for oral and injection use based on Thioctic Acid.
Common analogues of the product: Berlition 300 , Octolipen , Thioctacid BV , Thiolepta , Thiolipon , Lipothioxone , Thioctic acid-Vial , Thiogamma , Espa-Lipon , Neurolipon and so on.
In combination with cocarboxylase and riboflavin, the substance is part of the preparations Korilip-Neo and Korilip .
Multicomponent preparations: Turboslim , Bio-Max , Selmevit Intensive , Complivit Trimester (1st trimester, 2nd trimester and 3rd trimester).
Reviews
Reviews from doctors about alpha lipoic acid are mostly positive. The medicine is quite safe to use, rarely causes adverse reactions (when administered intravenously in large doses), patients tolerate it well, and the medicine is often prescribed as part of complex treatment in combination with other vitamins and medications.
There are a lot of reviews about Thioctic Acid for weight loss:
- “... I recently took a course of the drug. I followed a diet and exercised. I’ve lost weight, I’m very happy with everything”;
- “... Even as a child, a doctor prescribed this acid to me for the treatment of dyskinesia, and since then there have been almost no problems with gallstones. But sometimes I take this substance for prevention. I feel great”;
- “... After the course I always lose a couple of kilograms, I feel such lightness in my body, I no longer want to eat fatty and sweet foods”;
- “... I took the full course, spent money and time, went to shaping as usual, but didn’t see any results. Just a waste of money”;
- “... It’s good, of course, that the medicine is inexpensive and I didn’t have any adverse reactions from it, it’s a vitamin after all. But I can’t say that I actually lost weight because of it. The weight remained the same.”
Buy Thioctacid BV film-coated tablets 600 mg No. 30 in pharmacies
Tradename:
Thioctacid ® BV
INN or group name:
Thioctic acid
Dosage form:
Film-coated tablets
Compound:
1 film-coated tablet contains:
Active substance: thioctic acid (α-lipoic acid) – 600 mg Excipients: low-substituted hyprolose 157.00 mg, hyprolose 20.00 mg, magnesium stearate 24.00 mg. Film coating: hypromellose 15.80 mg, macrogol 6000 4.70 mg, titanium dioxide 4.00 mg, talc 2.02 mg, aluminum varnish based on quinoline yellow dye 1.32 mg, aluminum varnish based on indigo carmine 0.16 mg .
Description:
biconvex oblong tablets, film-coated, light green in color.
Pharmacotherapeutic group:
Metabolic agent.
Pharmacological properties
Pharmacodynamics
Thioctic acid is found in the human body, where it functions as a coenzyme in the oxidative phosphorylation reactions of pyruvic acid and alpha-keto acids. Thioctic acid is an endogenous antioxidant; according to its biochemical mechanism of action, it is close to B vitamins.
Thioctic acid helps protect cells from the toxic effects of free radicals arising in metabolic processes; it also neutralizes exogenous toxic compounds that have entered the body. Thioctic acid increases the concentration of the endogenous antioxidant glutathione, which leads to a decrease in the severity of symptoms of polyneuropathy. The drug has hepatoprotective, hypolipidemic, hypocholesterolemic, hypoglycemic effects; improves trophism of neurons. The result of the synergistic effect of thioctic acid and insulin is an increase in glucose utilization. Thioctacid® BV (fast release) is an optimized dosage form for oral administration, which avoids the high variability of thioctic acid concentrations in blood plasma.
Pharmacokinetics
When taking the drug orally, thioctic acid is quickly and completely absorbed from the gastrointestinal tract. Taking Thioctacid® BV simultaneously with food may reduce the absorption of thioctic acid. Taking the drug according to the recommendations 30 minutes before meals allows you to avoid unwanted interactions with food, since the absorption of thioctic acid is already complete at the time of eating. The maximum concentration of thioctic acid in the blood plasma is reached 30 minutes after taking the drug and is 4 mcg/ml. Thioctic acid has a “first pass” effect through the liver. The absolute bioavailability of thioctic acid is 20%. The main metabolic pathways are oxidation and conjugation. Thioctic acid and its metabolites are excreted by the kidneys (80-90%). The half-life is 25 minutes.
Indications for use
Diabetic and alcoholic polyneuropathy.
Contraindications
Hypersensitivity to thioctic acid or other components of the drug.
Pregnancy, breastfeeding period (there is no sufficient experience in using the drug).
There are no clinical data on the use of Thioctacid® 600 BV in children and adolescents; therefore, the drug cannot be prescribed to children and adolescents.
Directions for use and doses
The drug is used orally. The recommended dose is 1 tablet (600 mg) once a day. The drug is taken on an empty stomach, 30 minutes before breakfast, without chewing, with water.
In severe cases, treatment begins with the appointment of Thioctacid® 600 T solution for intravenous administration for 2 to 4 weeks, then the patient is transferred to treatment with Ti-octacid® BV.
Side effect
The incidence of side effects is determined as follows:
Very common: > 1/10; Often: 1/100; Uncommon: 1/1000; Rarely: 1/10000; Very rarely:
From the gastrointestinal tract:
Often – nausea; very rarely - vomiting, pain in the stomach and intestines, diarrhea, changes in taste.
Allergic reactions: Very rarely - skin rash, urticaria, itching, anaphylactic shock.
From the nervous system and sensory organs: Often – dizziness.
General:
Very rarely - due to improved glucose utilization, the level of glucose in the blood may decrease and symptoms of hypoglycemia may appear (confusion, increased sweating, headache, visual disturbances).
Overdose
Symptoms:
In the case of taking thioctic acid in doses of 10-40 g, serious signs of intoxication may be observed (generalized convulsive seizures; severe acid-base imbalance leading to lactic acidosis; hypoglycemic coma; severe blood clotting disorders, sometimes leading to fatal outcome) .
If a significant overdose of the drug is suspected (doses equivalent to more than 10 tablets for an adult or more than 50 mg/kg body weight for a child), immediate hospitalization is necessary.
Treatment: symptomatic, if necessary - anticonvulsant therapy, measures to maintain the functions of vital organs.
Interaction with other drugs
With the simultaneous administration of thioctic acid and cisplatin, a decrease in the effectiveness of cisplatin is observed. Thioctic acid binds metals, so it should not be administered concomitantly with drugs containing metals (for example, iron, magnesium, calcium). According to the recommended route of administration, Thio-ctacid® 600 BV tablets are taken 30 minutes before breakfast, while preparations containing metals should be taken at lunchtime or in the evening. For the same reason, during treatment with Ti-octacid® 600 BV, it is recommended to consume dairy products only in the afternoon.
With the simultaneous use of thioctic acid and insulin or oral hypoglycemic drugs, their effect may be enhanced, therefore regular monitoring of blood glucose levels is recommended, especially at the beginning of thioctic acid therapy. In some cases, it is permissible to reduce the dose of hypoglycemic drugs to avoid the development of symptoms of hypoglycemia. Ethanol and its metabolites weaken the effect of thioctic acid.
special instructions
Alcohol consumption is a risk factor for the development of polyneuropathy and can reduce the effectiveness of Thioctacid® BV, therefore patients should refrain from drinking alcoholic beverages both during treatment with the drug and during periods outside of treatment.
Treatment of diabetic polyneuropathy should be carried out while maintaining optimal blood glucose concentrations.
Release form
Film-coated tablets, 600 mg.
30, 60 or 100 tablets in a brown glass bottle with a capacity of 50.0, 75.0 or 125.0 ml, respectively, with a plastic cap with tamper evident.
1 bottle along with instructions for use in a cardboard box.
Storage conditions
At a temperature not exceeding 25° C, out of the reach of children.
Best before date
5 years. Do not use after the expiration date stated on the package.
Conditions for dispensing from pharmacies
On prescription.