The article was checked by an obstetrician-gynecologist, Ph.D. Sazonova Yu.M. , is for general informational purposes only and does not replace specialist advice. For recommendations on diagnosis and treatment, consultation with a doctor is necessary.
Itching and burning in the intimate area, which occur against a background of redness and inflammation, is the most common reason for visiting a gynecologist and dermatovenerologist. Before starting treatment, specialists at the Yauza Clinical Hospital establish the cause of this discomfort by conducting all the necessary laboratory tests. Etiotropic treatment (aimed at eliminating the cause) usually quickly leads to the elimination of the symptom.
Most often, such complaints arise with the development of vulvovaginitis and contact dermatitis. Itching and burning in the anogenital area not only disrupts an active life, but also contributes to the occurrence of pain during sex, and the appearance of these symptoms in postmenopause is a serious problem for a woman, which can sometimes lead to cancer.
Make an appointment with a gynecologist
The causative agent of the disease, human papillomavirus (HPV), belongs to the genus Papillomavirus, which, in turn, belongs to the family Papavaviridae. Human papillomaviruses are highly tissue specific and infect epithelial cells of the skin and mucous membranes. To date, more than 190 types of HPV have been identified and described, which are classified into high and low oncogenic risk groups according to their potential to induce cancer.
The International Agency for Research on Cancer identifies 12 types of high-risk HPV (types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59), which can potentiate the development of cancer and precancerous lesions of various locations: cervix uterus, vulva, vagina, anal canal, penis, neck, larynx, oral cavity.
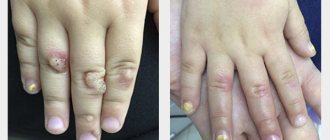
Anogenital warts are the most common clinical manifestation of human papillomavirus infection, with up to 90% of all cases of the disease in men and women caused by HPV types 6 and 11. The average time between HPV infection and the development of anogenital warts is 11-12 months in men and 5-6 months among women.
Human papillomavirus infection is most often recorded in young people who have a large number of sexual partners. According to WHO, 50-80% of the population is infected with HPV, but only 5-10% of infected individuals have clinical manifestations of the disease. The detection of HPV varies significantly in different ethno-geographical regions and is determined by behavioral, socio-economic, medical, and hygienic factors. Geographic variability is characteristic not only of the frequency of detection of the virus, but also of the distribution of HPV genotypes. According to a systematic analysis of global data, the incidence of anogenital warts in men and women (including new cases and relapses of the disease) varies from 160 to 289 cases per 100,000 population with an average of 194.5 cases per 100,000 population, and the average annual detection rate of new cases of anogenital warts is 137 cases per 100,000 population among men and 120.5 cases per 100,000 population among women.
In the Russian Federation, the incidence rate of anogenital warts in 2014 was 21.8 cases per 100,000 population: in persons aged 0 to 14 years - 0.6 cases per 100,000 population, in persons aged 15-17 years - 28.3 cases per 100,000 population, in persons over 18 years of age - 25.9 cases per 100,000 population.
However, these indicators do not reflect the true level of incidence and are a consequence of incomplete registration of new cases of anogenital warts. routes of infection
in adults: sexual contact.
In children:
- transplacental (rare);
- perinatal;
- sexual contact;
- contact-household, however, the possibility of autoinoculation and transmission of HPV through household objects remains insufficiently studied.
Anogenital (venereal) warts (genital warts)
Anogenital (viral) warts are a viral disease caused by the human papillomavirus and are characterized by the appearance of growths on the skin and mucous membranes of the external genitalia, urethra, vagina, cervix, and perianal area.
The causative agent of the disease, human papillomavirus (HPV), belongs to the genus papillomaviruses, which, in turn, belongs to the papavavirus family. Currently, there are more than 190 types of HPV, of which certain types are associated with diseases of the urogenital area, of which low risk varieties are identified - HPV 6 and 11, medium - HPV 31, 33, 35 and high oncogenic risk - HPV 16 and 18. In humans Those who are promiscuous often carry several types of viruses at once. A characteristic feature of this pathology is that patients are affected at a young age. HPV is considered as a possible etiological factor in squamous cell carcinoma of the cervix, vulvar and vaginal cancer.
The disease is transmitted primarily through sexual contact.
Factors contributing to the appearance or recurrence of HPV are: decreased immunological reactivity, hypothermia, intermittent diseases (mainly viral etiology), hormonal disorders. The appearance or recurrence of anogenital warts during pregnancy (due to its characteristic immunosuppression) and spontaneous regression after childbirth are observed.
The pathological process in men is localized on the inner and outer layers of the foreskin, the head of the penis, at the external opening of the urethra, the skin of the inguinal folds, the scrotum, and the perianal area. In women, anogenital warts most often affect the labia majora and minora, the clitoris, the skin of the external opening of the urethra, the inguinal folds, and the perianal area.
At first, single nodules the size of a pinhead, pink or grayish-red, appear, but over time their number increases. They grow in the form of papillae, often merging with each other, forming tumor-like growths reminiscent of cauliflower or raspberries. Anogenital warts have a soft consistency. The skin surrounding anogenital warts is usually unchanged. However, with constant mechanical irritation, the skin becomes bright red, itching and burning sensations appear.
Clinical diagnosis of anogenital warts is usually not difficult. Problems arise when diagnosed in the early stages of the disease, when anogenital warts are very small and resemble surface roughness. In this case, the main diagnostic method is cytological examination of biopsy specimens. The polymerase chain reaction method can be used for low-symptomatic or asymptomatic forms of the disease, as well as to determine the type of virus. In connection with the use of destructive methods in the treatment of anogenital warts, an additional serological test is carried out for syphilis, HIV, hepatitis B and C.
Consultations with other specialists are recommended according to indications in the following cases: - an obstetrician-gynecologist for the purpose of diagnosing background and dysplastic processes of the cervix, vulva and vagina; - urologist for intraurethral localization of anogenital warts; - proctologist if there is a process in the anal area; - immunologist in the presence of immunodeficiency conditions and recurrence of the disease.
The incubation period ranges from 3 weeks to 8 months, more often 2-3 months. The virus can remain latent throughout a person's life. Even with proper treatment and normal immunity, anogenital warts often recur. This is due to the persistence of the virus in the apparently healthy skin around the rash. Relapse is not associated with re-infection from a sexual partner, but with reactivation of the virus. Without treatment, anogenital warts may resolve on their own, remain unchanged, or progress.
The indication for treatment of anogenital warts is the presence of clinical manifestations of the disease. Since a complete cure for HPV infections cannot be achieved, the goal of therapy is to remove anogenital warts, and not to eliminate the pathogen. Treatment methods for anogenital warts are divided into the following main groups:
- Cytotoxic method
- Chemical method
- Immunomodulators for local use.
- Physical methods - electrocoagulation
- laser destruction
- cryodisruption
- Surgical excision
Using condoms reduces the risk of infecting sexual partners.
Brovkina I.V.
Genital warts: effective treatment methods
M.A.GOMBERG , Doctor of Medical Sciences, Professor, A.M. SOLOVIEV , Candidate of Medical Sciences, Associate Professor, N.I.CHERNOVA , Candidate of Medical Sciences, Associate Professor, S.G.ISAEVA , MGMSU, Moscow Infections caused by the human papillomavirus (HPV) are very widespread. In the United States alone, the Center for Disease Control (CDC) estimates that up to 5.5 million people per year are infected with HPV (Armstrong GL, et al., 2001).
In Russia, anogenital warts, the etiology of which is associated with HPV, are registered as sexually transmitted infections (STIs). The level of official registration of anogenital warts in Russia (about 35 per 100,000 population in recent years) differs significantly from the true prevalence of HPV infection.
Meanwhile, with the widespread introduction of molecular diagnostic methods into practice, doctors of various specialties are diagnosing HPV infection in a variety of clinical manifestations of papillomatosis outside the genital area. Often, HPV is detected without the presence of manifestations associated with this infection. This is a special case, and we discussed the tactics of managing such patients earlier (Gomberg M.A., Solovyov A.M. // Medical Council. - 2009. - No. 3. - P. 12-18).
The problem of HPV infection became especially relevant after the Nobel Prize in Medicine was awarded to Harald zur Hausen (Germany) in 2008 for proving the connection of cervical cancer with this particular virus. Currently, not only doctors, but also patients are aware of this.
The purpose of this article is to give outpatient doctors an idea of modern approaches to the management of patients with HPV infection, so that when anogenital warts are detected in patients, they can solve this problem on their own, without referring the patient to specialized centers.
Principles of treatment of clinical manifestations of HPV
According to existing principles for the management of patients with genital clinical manifestations associated with HPV, treatment is aimed at either destroying papillomatous lesions that arise at the site of virus entry by one method or another, or stimulating an antiviral immune response, or a combination of these approaches. The most optimal is the following classification of methods for treating anogenital warts: I. Destructive methods: 1. Physical - surgical excision; — electrosurgical methods; — cryotherapy; - laser therapy. 2. Chemical: - nitric acid; - trichloroacetic acid; - solcoderm. II. Cytotoxic drugs: - podophyllin (PF); — podophyllotoxin (PFT); - 5-fluorouracil. III. Immune methods: - interferons; — interferon inducers; - immunotropic drugs. IV. Combined methods: - combined use of various methods, usually immune and destructive. When choosing the most optimal method in each specific case, the doctor must be guided by the following main characteristics: - effectiveness for a given pathology; — frequency of relapses after treatment; — tolerability (minimal side effects); — simplicity of procedures.
Treatment of genital clinical manifestations associated with HPV is aimed either at destroying, by one method or another, papillomatous lesions that arise at the site of virus introduction, or at stimulating an antiviral immune response, or at a combination of these approaches
The main difference between physical destructive methods is that when they are used, rapid, often instantaneous destruction of lesions occurs. When using other methods, the lesions are eliminated within a few hours or weeks. As a rule, this time is comparable to the healing time of lesions after exposure to physical methods. The frequency of relapses, according to the literature, does not depend on the choice of treatment method.
Thus, the obvious advantages of physical methods are neutralized. To use physical destructive methods, special premises, expensive equipment, and trained personnel with certificates for this type of medical activity are required. All this limits the use of these methods in widespread practice, especially in small medical centers or in areas where the clinic has only dermatovenerological, gynecological or urological offices. In this regard, conservative treatment methods that can be applied by any practitioner are of particular interest.
Chemical destructive methods
Concentrated solutions of acids, alkalis, salts, etc. are used, for example, feresol, hydrogen peroxide, solutions of quinine and hingamine, preparations based on salicylic and lactic acids, acetic and nitric acid, thuja and celandine juices. All these methods have low, poorly predictable effectiveness, and numerous side effects. There is little consistent data on the effectiveness of these drugs.
Feresol
Feresol is a solution containing 60% phenol and 40% tricresol. It is used 1-2 times with an interval of 3-4 minutes once a week. Can be repeated 3-4 times. Among the chemicals that have a destructive effect, about which there is sufficient data in the literature, it should be noted that the combined acid preparation is solcoderm.
Solcoderm
Solcoderm is an aqueous solution, the active component of which is the interaction products of organic acids (acetic, oxalic and lactic) and metal ions with nitric acid having a concentration of 6.6 N. The solution contains nitrites in an amount of 0.02 mg/ml. When applied topically to affected areas, solcoderm leads to intravital fixation (the structure of the neoplasm is preserved) followed by mummification of the pathologically altered tissue with which the drug comes into contact. Healing occurs under the scab, which eliminates the formation of an open wound surface. Complications of treatment, such as secondary infection or scarring, according to the literature, are extremely rare. Ease of use, effectiveness in removing diseased tissue with minimal negative impact on surrounding tissue, and the ability to control the extent of treatment make Solcoderm useful for treating a wide range of benign skin changes.
The uniqueness of Solcoderm is that the acids included in its composition, having a low concentration and, therefore, a low probability of side effects, have a pronounced clinical effect due to redox reactions. The nitrogen oxidation products that are formed as a result of these reactions have some additional clinical benefits, characterized by more complete destruction of the affected area and reduced damage to surrounding healthy tissue (Weiner M., et al., 1983).
We observed 24 patients (14 men and 10 women) aged from 17 to 41 years (average age 25.8 years): 16 patients with genital warts on the genitals, 3 patients with vulgar warts on the hands, 2 patients with plantar warts and 3 with smooth skin nevi. Solcoderm was applied with a special plastic spatula or using a glass capillary onto the surface of the lesion, previously degreased with alcohol. The fastest way to saturate the lesion was achieved by applying the solution using capillary tubes, followed by mechanical action with a plastic spatula for deeper penetration of the solution.
Treatment of warts was carried out until yellow staining of lesions located on the skin or white staining of lesions located on the mucous membranes appeared. The appearance of a uniform yellow or white color indicates the sufficiency of processing and is a guarantee of subsequent mummification of the formation. A control examination of patients was carried out 3-5 days after primary treatment. If necessary, the lesions were re-treated with Solcoderm.
In patients with genital warts and nevi, in most cases, a single treatment with Solcoderm was sufficient. Complete rejection of the scab occurred within a period of 5 (usually with condylomas) to 14 (in the case of nevi) days, depending on the location and size of the lesions. For the treatment of vulgar warts, 2-3 therapy sessions were required, for the treatment of plantar warts - 3-4 sessions. In all cases, it was possible to achieve complete disappearance of the lesions. The treatment was well tolerated and no serious side effects were noted. During a 6-month follow-up, disease recurrence was noted in 3 patients with genital warts.
The data we obtained about the high effectiveness and good tolerability of Solcoderm therapy completely coincides with the data of large foreign studies. In the treatment of common and plantar warts, the drug was effective in 85-94% of patients (the recurrence rate was 6-10%) (Hettich R., Burri P., Binet O., 1984). In the treatment of genital warts in men and women, these figures were 80.1 and 7.0%, respectively, and in 64.7% of patients the effect was achieved after a single treatment (Brokalakis J., et al., 1984).
The short duration of treatment, the outpatient nature of the treatment and the precision with which the drug is applied to pathological tissues make Solcoderm a successful drug for the treatment of genital warts, as well as common and plantar warts.
Cytotoxic drugs. Podophyllin
Podophyllin is a resin obtained from the plants P. pelatum and P. emodi, native to North America and the Himalayas. To treat warts of the anogenital area, a 10-25% solution of podophyllin in ethanol or benzoin tincture is used. Podophyllin is the drug of choice in UK clinics (Reynolds M., et al., 1993). It binds to the microtubule apparatus of the cell and inhibits mitosis, and also suppresses the transport of nucleic acids, resulting in inhibition of DNA synthesis and cell reproduction (Handley JM, et al., 1994). Podophyllin is not registered in Russia. Some researchers oppose the use of the drug, especially by patients themselves. In particular, Petersen, et al. (1995) consider podophyllin to be a poorly studied, crudely purified plant extract. Using liquid chromatography, these authors determined that approximately 10% of the dry matter of a 20% podophyllin solution consists of 2 mutagenic flavonoids - quercetin and kaemferol. In this regard, it is proposed to use only highly purified podophyllotoxins, but their independent use can be recommended to patients only after detailed instructions.
Podophyllotoxin
Podophyllotoxin is the most therapeutically active fraction of podophyllin. Available in the form of 0.25, 0.3 and 0.5% solutions, as well as in the form of 0.15, 0.3 and 0.5% cream.
Podophyllotoxin solution and cream are registered in Russia: - condiline - 0.5% solution. Apply 2 times/day for 3 days, then break for 4 days. The duration of treatment is no more than 5 weeks. — Vartek – 0.15% cream.
The undoubted advantage of podophyllotoxin is the possibility of safe self-administration of the drug by patients. Podophyllotoxin is used 2 times a day for 3 consecutive days a week for 4-5 weeks.
For podophyllotoxin, the effectiveness rate is 26-87% in men and 50-77% in women (Lassus A., 1987; Von Krogh G., 1987; Edwards A., et al., 1988; Kirby P., et al., 1990; Baker DA, et al., 1990; Handley JM, et al., 1991; Greenberg MD, et al., 1991). The wide range of effectiveness indicators is due to different periods of observation of patients, as well as the fact that in some studies the authors do not take relapses into account. The most common side effects of podophyllotoxin use according to Bonnez W., et al. (1994) are local inflammatory reactions (in 57% of patients), erythema, burning (in 48%), soreness (in 47%), itching (in 44%), slight weeping and erosion in the area of application (in 39%). Although systemic side effects have not been described, it is recommended to use podophyllotoxin in an amount of no more than 0.2 ml per procedure (Von Krogh G., 1982).
Handley JM, et al. (1994) considers podophyllotoxin to be the drug of choice for self-administration by patients with a small number of non-keratinizing condylomas located on the foreskin, glans penis, coronary sulcus and vulva. The drug is ineffective in the treatment of warts of the perianal area, vagina and urethra.
The disadvantages of podophyllotoxin are its high cost, long duration of treatment combined with not the highest efficiency.
5-Fluorouracil
5-fluorouracil is a pyrimidine antagonist and is capable of disrupting the synthesis of both cellular and viral DNA. For the treatment of warts of the anogenital area, it is prescribed in the form of a 5% cream (Krebs HB, 1987). According to Handley, et al. (1994), 5-fluorouracil is an effective treatment for intravaginal warts and condylomas of the terminal urethra. When treating intravaginal warts, the drug is prescribed once a night for a week or once a week for 10 weeks (Krebs HB, 1987). Although the drug with this treatment regimen has a fairly high effectiveness (85-90%), its use may cause weeping erosions on the vaginal mucosa, up to the development of severe weeping contact dermatitis.
When treating warts of the terminal part of the urethra, the cream is administered immediately after urination, at night for 3-8 days. Complete cure of intraurethral warts is observed in 90-95% of men (Dretler SP, et al., 1975; Von Krogh G., 1976). However, during treatment there are many side effects: stenosis and stricture of the urethra, dysuria, ulceration (Krebs HB, 1987).
Despite the fairly high effectiveness of 5-fluorouracil, availability and low cost, its use in widespread practice is limited due to the high frequency of side effects. The drug is contraindicated during pregnancy.
There are no official preparations of fluorouracil for external use in Russia, but a cream of the required concentration can be prepared according to a prescription in the pharmacy production department from the substance.
Immune methods. Interferon
Since HPV persists in epithelial cells and the use of destructive methods does not guarantee against relapses, the use of interferons both as monotherapy and in combination with other treatment methods is promising in this regard.
It has been established that when interferon is used in patients, the amount of viral DNA in the lesions decreases (according to PCR data), which correlates with clinical improvement or disappearance of the lesions (Arany I., et al., 1995).
Interferon in the treatment of anogenital warts can be used locally, intralesional and systemically (subcutaneously, intramuscularly, intravenously or rectally).
Most studies have shown low effectiveness of external use of interferon (Keay S., et al. 1988). Gross G. (1996) believes that an important indication for external use of interferon is the presence of subclinical foci of HPV infection and CIN (especially caused by HPV types of high oncogenic risk).
According to various authors, with systemic use of interferon alpha in a dose of 1.5 to 3 million IU intramuscularly or subcutaneously every other day for 4 weeks, 11-100% of patients experience complete disappearance of warts (Gross G., et al., 1986; Zwiorek L., et al., 1989).
Flu-like symptoms may occur with systemic use of interferon, the severity of which depends on the dose received (Handley, et al., 1994). These side effects can be reduced by prescribing non-steroidal anti-inflammatory drugs.
Considering that the effectiveness of systemic interferon monotherapy is low and not predictable enough, and also taking into account the high cost of treatment, this method is not widely used in clinical practice.
According to various authors, intralesional use of alpha and beta interferon is most effective. This method of therapy leads to the disappearance of 35-62.5% of warts, both treated and untreated (Corwin Vance J., et al., 1986; Reichman RC, et al., 1988; Welander CE, et al., 1990). The Russian market offers a wide selection of interferons from various manufacturers, both domestic and foreign - Viferon, Kipferon, Reaferon, Roferon-A, Intron A, Realdiron, etc.
It is preferable to use recombinant rather than human interferons. Drugs registered for the treatment of human papillomavirus infection include:
Viferon is a recombinant interferon alpha-2b. Available in the form of ointment, gel and suppositories. For papillomavirus infection, suppositories are used at a dose of 500,000 IU 2 times a day for 5-10 days;
Intron A – recombinant interferon alpha-2b. Apply systemically;
Roferon A is recombinant interferon alpha-2a. Apply subcutaneously at 1-3 million IU 3 times a week for 1-2 months;
Altevir is a recombinant interferon alpha-2b. Apply systemically;
Wellferon is purified interferon alpha-n1. Apply subcutaneously at 5 million IU daily for 14 days, then 3 times a week for 6-8 weeks.
There are reports of the effective use of interferon inducers as monotherapy. Of interest is the local use of a low molecular weight derivative of imihidazoquinolinamine - imiquimod, which is an inducer of cytokines and, in particular, alpha-interferon (Baker D., et al., 1995; Trofatter KE, 1997). It is used in the form of a 5% cream 3 times a week or daily at night until the rash completely disappears (but not more than 4 months). Complete disappearance of condylomas is observed in 56% of patients with the first dosage regimen and in 71% with daily use (placebo - 14%).
At follow-up examinations within 1 year, relapses were observed in only 13-19% (Baker D., et al., 1995; Trofatter KE, 1997). With daily use, local side effects more often developed: redness, swelling, erosion. The cream is especially effective in the treatment of subclinical HPV infection (Gross G., 1996). Imiquimod has been widely used in Europe, the USA, Japan and many other countries since the late 90s of the 20th century, but this drug has not yet been registered in Russia.
Relapse Prevention
To prevent relapses of HPV infection, it is more preferable to use interferon or its inducers, as well as other activators of antiviral immunity as adjuvant therapy in combination with various destructive methods. Immune drugs increase the effectiveness of treatment and reduce the frequency of relapses. Combination immunotherapy is of particular importance in the treatment of stubborn, difficult-to-treat warts.
Various methods have been proposed for treatment based on the use of immune drugs in combination with cryotherapy, laser therapy, electrocoagulation, solcoderm, etc. (Thin N., 1995). For example, it was found that the combination of interferon with laser therapy is more effective than laser therapy alone: complete disappearance of warts is observed in 52 (81.5%) and 19 (61%) patients, respectively (Corwin Vance J., et al., 1990; Hohenleuter U., et al., 1990; Petersen CS, et al., 1991).
Supplementing CO2 laser excision with low-dose alpha-interferon therapy (1-3 million IU per day for 5-7 days, then a break of 3-4 weeks, a course of 3-4 cycles) increases the effectiveness of treatment and reduces the percentage of relapses observed after the use of laser therapy only (Gross G., 1996). For small genital warts, the addition of laser therapy or electrocoagulation with local application of a gel containing interferon beta 0.1 million IU/g, 5 times a day for 4 weeks, effectively prevents relapses (Gross G., 1996).
However, according to The Condylomata International Collaborative Study Group (1993), the use of interferon alpha (3 times a week for 4 weeks) after removal of all visible condylomas with a CO2 laser does not give any positive results in comparison with placebo (complete cure occurred in 18%, relapses occurred in 35% of patients). According to Hopel MR, et al. (1992), the combination of the use of alpha interferon (3-6 five-day courses with an interval of 2 weeks) with destructive and/or surgical methods, despite increasing the effectiveness of treatment of persistent, treatment-resistant condylomas, does not prevent the development of relapses that occur in 50% of patients .
To prevent relapses of HPV infection, it is more preferable to use interferon or its inducers, as well as other activators of antiviral immunity as adjuvant therapy in combination with various destructive methods
Another group of conservative methods for treating HPV infection is of interest - these are immune drugs that can be used alone or in combination with destructive methods.
Immunotropic drugs
Antiviral immunity can be influenced by using inducers of endogenous interferon and other immune activators. There are many immunoactive drugs on the Russian pharmaceutical market, which, according to the manufacturer’s instructions or the recommendations of researchers, can be used in the combined treatment of anogenital warts in combination with various destructive methods.
Gepon
Gepon is a synthetic oligopeptide consisting of 14 amino acid residues. The drug belongs to the group of immunomodulators and is indicated to increase the effectiveness of immune defense against infections, treatment and prevention of opportunistic infections caused by bacteria, viruses or fungi.
The immunopharmacological and antiviral effects of the drug Gepon are due to the fact that it: - causes the production of alpha and beta interferons; — mobilizes and activates macrophages; - limits the production of inflammatory cytokines (interleukins 1, 6, 8 and tumor necrosis factor); - stimulates the production of antibodies to various antigens of an infectious nature; — suppresses viral replication; – increases the body’s resistance to viral and bacterial infections.
In patients with a weakened immune system, Gepon: - increases the reduced content of CD4+ T- and NK-cells; — increases the functional activity of neutrophils and CD8+ T cells, which are key parts of the body’s defense against bacteria, viruses and fungi; - enhances the production of antibodies specific to pathogens of opportunistic infections that are relevant for a given patient; - prevents the development of relapses of opportunistic infections.
Although the instructions for use of the drug do not directly indicate its use for papillomavirus infection (the drug is recommended to increase immune protection against infections, treatment and prevention of opportunistic infections caused by bacteria, viruses or fungi), the accumulated clinical experience of its use allows us to recommend Gepon for HPV. For example, applications of Gepon to the foci of HPV lesions after their removal by any destructive method (from 3 to 6 applications every other day) can speed up the healing process and reduce the level of recurrence (Soloviev A.M., 2003). Systemically, the drug is used at a dose of 2 mg orally 3 times a week. The course can be repeated at intervals of 1 week.
Immunomax
The use of an antiviral immunity activator, Immunomax, which is an acidic peptidoglycan with a molecular weight of 1000-40000 kDa, is promising. The drug is isolated from plants using a complex of biochemical methods, including ultrafiltration and chromatography. The sterile drug Immunomax is available in the form of a lyophilized powder of 200 units in vials for injection.
There is data on the use of the drug for clinical manifestations of HPV infection in combination with any of the destructive methods. Patients have genital warts removed and at the same time given intramuscular injections of Immunomax 200 units once a day on the 1st, 2nd, 3rd, 8th, 9th and 10th days of treatment (Perlamutrov Yu.N. et al., 2003). In 68% of patients, immediately after treatment, there is no recurrence of condylomas, and after additional destruction sessions, the effectiveness of treatment reaches 98% (with a follow-up of at least 3 months).
Epigen-intim
Epigen-intim is an aerosol preparation of plant origin for topical use, the main active ingredient of which is glycyrrhizic acid, obtained from licorice root. The drug activates local immunity due to interferonogenic action, while increasing the ability of tissues to protect against infections. Glycyrrhizic acid, by inhibiting DNA and RNA viruses, causes their inactivation, blocks the introduction of active viral particles through the membrane into the cell, and disrupts the synthesis of new structural components of viruses. The main mechanism of the antiviral activity of glycyrrhizic acid is inhibition of P kinase, which entails inhibition of phosphorylation of cellular and virus-encoded proteins in infected cells and those in the free state.
It has been shown that the use of epigen in combination therapy of anogenital warts can reduce the frequency of relapses (Chernova N.I., 2004). The drug also has an anti-inflammatory and healing effect, which is important when treating condylomas with destructive methods.
When used periodically for a long time in patients with asymptomatic HPV shedding, epigen has a preventive effect in relation to the clinical manifestation of infection - during observation for 1 year, condylomas appeared in 14.7%, while in the control group this occurred in 40% of patients (Chernova N.I., 2004).
Epigen-intim is used intravaginally, externally and intraurethrally. For external use, the drug is applied to the entire affected surface from a distance of 4–5 cm using 1–2 valve presses. The drug is injected intravaginally by 1-2 clicks on a special nozzle. The drug is used before removal of condylomas 3 times a day, against the background of destruction - 5 times a day for 10 or more days until healing; for the prevention of immediate relapses - 3 times a day for 1 month.
Immunotropic drugs with direct antiviral action
Immune drugs increase the effectiveness of treatment and reduce the frequency of relapses
Isoprinosine (inosine pranobex)
Isoprinosine (inosine pranobex) is an antiviral agent with immunomodulatory properties. The drug normalizes the deficiency or dysfunction of cellular immunity, inducing the maturation and differentiation of T lymphocytes and T1 helper cells, potentiating the induction of a lymphoproliferative response in mitogenic or antigen-active cells. Isoprinosine models the cytotoxicity of T-lymphocytes and natural killer cells, the function of T8 suppressors and T4 helpers, and also increases the amount of immunoglobulin G and surface complement markers. Isoprinosine increases the synthesis of interleukin-1 (IL-1) and interleukin-2 (IL-2), regulates the expression of IL-2 receptors, significantly increases the secretion of endogenous interferon-β and reduces the production of interleukin-4 in the body.
The drug enhances the effect of neutrophil granulocytes, chemotaxis and phagocytosis of monocytes and macrophages. Isoprinosine also has a direct antiviral effect, inhibiting the synthesis of viruses by incorporating inosine-orotic acid into the polyribosomes of the virus-affected cell and disrupting the attachment of adenylic acid to viral RNA.
One of the indications for the use of the drug is infections caused by the human papillomavirus: genital warts, human papillomavirus infection of the vulva, vagina and cervix (as part of complex therapy).
For infections caused by HPV, isoprinosine is prescribed 3 g/day (2 tablets 3 times/day) as an adjunct to local therapy or surgery for 14–28 days in low-risk patients or 5 days a week consecutively for 1–28 days. 2 weeks per month for 3 months in high-risk patients.
The literature describes various schemes and results of the use of isoprinosine for papillomavirus infection. There is known foreign experience in the use of inosine pranobex as a therapy that complements the destruction of anogenital warts (Mohanty KC, et al., 1986). The drug was used at a dose of 1 g 3 times a day for 28 days. The effectiveness of therapy increased from 41 to 94% when isoprinosine was added to traditional methods of treatment.
Similar results are shown in the works of V.N. Prilepskaya. et al. (2007). Adding isoprinosine to standard treatment at a dose of 1 g 3 times a day for 5 days before destruction increases the effectiveness of therapy from 65.6% to 87.5%, and reduces the frequency of relapses by 3 times. There is also a high frequency of HPV elimination from lesions - 65.6%.
In the work of Zabelev A.V. et al. (2005) showed the disappearance of atypical epithelium after courses of isoprinosine in women with HPV-associated low-grade squamous intraepithelial lesions. Isoprinosine was prescribed 1 gram 3 times a day for 5 days, 3 courses with an interval of 1 month.
Similar results - an improvement in the morphological picture of the vulvar epithelium - were shown in a study by Sun Kuie Tay (1996). Inosine pranobex was prescribed 1 g 3 times a day for 6 weeks. A positive effect was achieved in 63.5% of patients, and in the group taking placebo – in 16.7%. To monitor and process research results from the standpoint of evidence-based medicine, the Astra program was developed and implemented in 2008 - an all-Russian multicenter program for monitoring, summarizing and generating statistical reporting on the features and results of the use of isoprinosine in the treatment of diseases associated with HPV in normal conditions. medical practice. The study involved 6191 patients (5896 women and 295 men) (Kostava M.N. et al., 2009).
Patients who took part in the study were treated in accordance with the identified diseases; the drug isoprinosine was included in the treatment complex. In the presence of only genital warts of the vulva and vagina, monotherapy with isoprinosine was performed. Treatment of CIN I-II, localized in a limited area of the exocervix, fully accessible for colposcopy, without damage to the cervical canal in 58 patients was also carried out with the drug isoprinosine. In the absence of a positive effect, adequate excision of the affected tissue was performed. Recommended therapeutic doses of isoprinosine corresponded to the severity of epithelial damage by the human papillomavirus. When genital warts were detected in the vulva and vagina, isoprinosine was prescribed at a dose of 50 mg/kg per day for 5 days in three courses with a break of a month. For cytological and histological data corresponding to CIN I–II, isoprinosine was used at a dose of 50 mg/kg/day, 10 days a month in 3 courses with a break of a month. For subclinical forms of HPV lesions of the epithelium, the drug was prescribed at a dose of 50 mg/kg/day, the course of treatment was from 10 to 21 days.
Before treatment, koilocytosis with a normal colposcopic picture was detected in 1367 patients, and after treatment - only in 71. Treatment of genital warts was effective in 91% of patients, combined treatment of CIN I - in 90%, combined treatment of CIN II - in 82%, treatment subclinical form of HPV infection - in 91% [Kostava M.N. et al., 2009].
Recently, a meta-analysis of the results of using isoprinosine was conducted (Eliseeva M.Yu. et al, 2009). Literature data based on 15 sources, which describe 2369 cases of the use of isoprinosine as monotherapy, 3369 in combination with other methods, compared with 71 observations of placebo control and 575 observations of traditional treatment, indicate the effectiveness of auxiliary immunotherapy with isoprinosine against the background of traditional methods for the treatment of genital lesions associated with HPV.
The main indicator of the effectiveness of any method of removing anogenital warts is the absence of relapses. Since the frequency of relapses of HPV infection does not depend on the method of destruction of lesions (physical, chemical or cytotoxic), destruction methods that can be used directly in the office are of particular interest to a doctor at an outpatient appointment. Therefore, in this work, we tried to focus on methods that allow us to quickly and effectively combat the manifestations of HPV infection and its relapses without the use of expensive methods of destruction, access to which is not available in every specialized clinic.
The advantage of this treatment is that there is no risk of infecting the doctor with viral particles, in contrast to laser destruction without the doctor using protective methods. To prevent relapses of HPV infection, it is recommended to prescribe immunotropic therapy. This approach will provide effective treatment of anogenital warts, which should significantly affect all aspects of the control of HPV infection in the population, including the level of its registration.
The main indicator of the effectiveness of any method of removing anogenital warts is the absence of relapses. Since the frequency of relapses of HPV infection does not depend on the method of destruction of lesions (physical, chemical or cytotoxic), in this work we tried to focus on methods that allow us to quickly and effectively combat the manifestations of HPV infection and its relapses. To prevent relapses of HPV infection, it is recommended to prescribe immunotropic therapy. This approach will provide effective treatment of anogenital warts, which should significantly affect all aspects of the control of HPV infection in the population, including the level of its registration.
It should be recognized that even modern advances in the research of human papillomavirus infection and the improvement of diagnostic and therapeutic methods leave a number of unresolved issues - the lack of standards for HPV therapy, the widespread dissemination of recommendations for patients on the prevention of HPV infection, and algorithms for the management of pregnant women with HPV.
Prevention
To avoid the development of diseases associated with HPV, it is recommended:
- Use condoms during sexual intercourse.
This significantly reduces the risk of contracting genital warts. - Get vaccinated.
The drug Gardasil protects against four strains of HPV that cause cancer and is used to prevent genital warts. Another vaccine, Cervarix, protects against cervical cancer but not genital warts.
Routine HPV vaccination is recommended for girls and boys aged 11 and 12 years. If vaccinations were not given in childhood, it is recommended that girls and women under 26 years of age, and boys and men under 21 years of age, receive the vaccine.
The drugs are effective if given before they become sexually active. Research has shown that people under the age of 21 and from 21 to 30 years of age who have received the HPV vaccine are 50% protected from infection.
Side effects from vaccines are minor and include soreness at the injection site (shoulder), headaches, low fever, or flu-like symptoms. Sometimes dizziness or fainting occurs after the injection, especially in teenagers.
Warts of the anogenital area: causes of relapses and how to combat them
26.12.2018
1158
1
Anogenital warts are a manifestation of a disease caused by the human papillomavirus (HPV) and characterized by the appearance of exophytic and endophytic growths on the skin and mucous membranes of the external genitalia, urethra, vagina, cervix, and perianal area. HPV belongs to the genus Papillomavirus. These viruses are highly tissue specific and are classified into high and low oncogenic risk groups according to their potential to induce malignant growth [5, 6].
With anogenital warts, in 45% of cases, highly oncogenic types of human papillomavirus infection (PVI) are found, which can initiate cervical cancer and other precancerous and cancerous lesions of the genital organs [8]. In recent years, the role of HPV in the development of bladder and prostate cancer has been discussed [9]. In Russia, anogenital warts are registered as sexually transmitted infections [8].
The problem is quite common. About 32 million new cases of the disease are registered annually in the world. In the United States, the incidence has increased 6-fold over the past 40 years [11]. In Europe, the highest incidence rate was noted in the UK - 120 cases per 100 thousand population [3, 17]. In the Russian Federation, the incidence rate of anogenital warts in 2014 was 21.8 cases per 100 thousand population, which differs significantly from the true prevalence of PVI [6, 8].
The main route of transmission of PVI in adults is sexual [12, 16]. In children, transplacental, perinatal and household contact routes of infection are possible [6]. Clinical types of anogenital warts vary from genital warts and papules to manifestations of intraepithelial neoplasia [13]. The very presence of anogenital warts is a direct indication for treatment [14, 16].
The goal of treating HPV manifestations is: 1) destruction of anogenital warts; 2) improving the quality of life of patients [6, 16]. And if the implementation of the first point in modern medicine does not pose a problem (a lot of effective minimally invasive methods for removing anogenital warts have been proposed), then with the improvement in the quality of life of patients with PVI, not everything is so favorable, and this is largely due to relapses of clinical manifestations of HPV [17]. For example, in 2021 in England, according to sexual health services, 122 thousand episodes of genital warts were identified, of which 49% were diagnosed as relapses [1].
The tendency to relapse and persistence of PVI is determined mainly by the high stability of the patient’s immunity [19]. Moreover, the persistence of the virus in the body is associated to a greater extent with systemic immune defense, while relapses of clinical manifestations of HPV are determined by local immunoreactivity at the “problem” point [18]. These statements are supported by the fact that the manifestation of HPV infection often occurs when the integrity of the skin and mucous membranes is violated, the appearance of repeated lesions in areas of previous destruction of condylomas and in HIV infection accompanied by systemic immunosuppression [7].
Risk factors are disorders of cellular immunity, interferon and cytokine status, chronic somatic diseases (diabetes mellitus, oncohematological processes, etc.), mixed viral-bacterial infection, as well as increasing age [9, 11]. Also, variants of the course of PVI may be associated with genetic and phenotypic heterogeneity in terms of resistance to HPV. Thus, in individuals with HLA DQW3 genes, “weak” genes of the immune response to HPV are linked, which contributes to the chronicity of the infection, and in individuals with other types of HLA, the genes of which are linked to “strong” genes of the immune response, a transient infection develops, this can also explain absence of infection in some sexual partners in discordant couples [2, 8].
How to deal with recurrence of HPV? A modern approach to the treatment of diseases should be carried out in accordance with the standards adopted for this nosology, developed on the basis of the principles of evidence-based medicine. In the “Federal Clinical Guidelines (FCR) for the management of patients with anogenital (venereal) warts” there is a group of drugs that deserve attention precisely in the light of limiting relapses and persistence of PVI, namely “Immunomodulators for local use” [4, 6].
FCRs recommend the first line of therapy to use imiquimod cream (registered in Russia under the trade name “ Vartotsid ”), as a non-invasive agent that can be easily used by patients without medical supervision [4, 6, 10]. The cream is applied in a thin layer to anogenital warts at night (for 6-8 hours) 3 times a week (every other day). In the morning, the cream must be washed off the skin with warm water and soap. The course of treatment is continued until the anogenital warts disappear, but not more than 16 weeks [4].
The effectiveness and safety of this type of therapy was assigned a high level of evidence - A, based on data from meta-analyses, systematic reviews, rated with maximum scores and demonstrating the stability of the results [6]. Also, intralesional administration of α-interferon drugs is recommended as an alternative treatment regimen for FCR (level of evidence – B) [6, 8].
The use of systemic interferons (interferon gamma 500,000 IU, administered subcutaneously, 1 time per day, every other day, for a course of 5 injections) and inosine pranobex orally is recommended, according to the FKR, for severe recurrent HPV infection (level of evidence - A) [ 6, 11]. A combination of drugs for systemic action and local immunomodulators is more effective in reducing the likelihood of recurrence and further persistence of PVI, and must be used if treatment with one of the components fails [6].
Bibliography
- Public Health England. Sexually transmitted infections (STIs): annual data tables. Table 1: STI diagnoses and rates in England by gender, 2008 to 2017. https://www.gov.uk/government/statistics/sexually-transmitted-infections-stis-annual-data-tables. Accessed 14 Dec 2021.
- Kitsak V.Ya. “HPV-negative” and HPV-negative cervical cancer: the trigger role of HPV of high carcinogenic risk and alternative etiological factors / V.Ya. Kitsak // Bulletin of postgraduate medical education. - 2009. - No. 1. - P. 82-83.
- Desai S, Wetten S, Woodhall SC, Peters L, Hughes G, Soldan K. Genital warts and cost of care in England. Sex Transm Infect. 2011;87:464–8.
- Smirnov V. S., Kudryavtseva T. A. Vartotsid (imiquimod). – St. Petersburg: Hippocrates, 2021. – 144 p.
- Komericki P, Akkilic-Materna M, Strimitzer T, Aberer W. Efficacy and safety of imiquimod versus podophyllotoxin in the treatment of anogenital warts. Sex Transm Dis. 2011;38(3):216–8.
- “Federal clinical guidelines for the management of patients with anogenital (venereal) warts.” Moscow. - 2015. - P.3-11.
- Moore RA, Edwards JE, Hopwood J, Hicks D. Imiquimod for the treatment of genital warts: a quantitative systematic review. BMC Infect Dis. 2001;1:3.13. Arany I, Tyring SK, Stanley MA, Tomai MA, Miller RL, Smith MH, et al. Enhancement of the innate and cellular immune response in patients with genital warts treated with topical imiquimod cream 5%. Antivir Res. 1999;43:55–63.
- Fayzullina E.V. Clinical and organizational aspects of medical care for patients with anogenital warts as the most important factor in preserving the reproductive health of the population / E.V. Fayzullina, D.V. Frizin, L.K. Bunakova // Practical Medicine. - 2012. - No. 9 (65). — pp. 170-174.
- Kirby P, Dunne A, King DH, Corey L. Double-blind randomized clinical trial of self-administered podofilox solution versus vehicle in the treatment of genital warts. Am J Med. 1990;88(5):465–9.
- Edwards L, Ferenczy A, Eron L, Baker D, Owens ML, Fox TL, et al. Self-administered topical 5% imiquimod cream for external anogenital warts. HPV study group. Human PapillomaVirus. Arch Dermatol. 1998;134(1):25–30.
- Beutner KR, Spruance SL, Hougham AJ, Fox TL, Owens ML, Douglas JM Jr. Treatment of genital warts with an immune-response modifier (imiquimod). J Am Acad Dermatol. 1998;38(2 Pt 1):230–9.
- Arican O, Guneri F, Bilgic K, Karaoglu A. Topical imiquimod 5% cream in external anogenital warts: a randomized, double-blind, placebo-controlled study. J Dermatol. 2004;31(8):627–31.
- Garland SM, Waddell R, Mindel A, Denham IM, McCloskey JC. An open-label phase II pilot study investigating the optimal duration of imiquimod 5% cream for the treatment of external genital warts in women. Int J STD AIDS. 2006;17(7):448–52.
- Fife KH, Ferenczy A, Douglas JM Jr, Brown DR, Smith M, Owens ML, et al. Treatment of external genital warts in men using 5% imiquimod cream applied three times a week, once daily, twice daily, or three times a day.Sex Transm Dis. 2001;28(4):226–31.
- Grillo-Ardila CF, Angel-Muller E, Salazar-Diaz LC, Gaitan HG, Ruiz-Parra AI, Lethaby A. Imiquimod for anogenital warts in non-immunocompromised adults. Cochrane Database Syst Rev. 2014;11:CD010389.
- Lacey CJ, Woodhall SC, Wikstrom A, Ross J. 2012 European guideline for the management of anogenital warts. J Eur Acad Dermatol Venereol. 2013;27(3):e263–70.
- British Association for Sexual Health and HIV. United Kingdom National Guidelines on the Management of Anogenital Warts 2015. London: British Association for Sexual Health and HIV; 2015.
- Schofer H, Van Ophoven A, Henke U, Lenz T, Eul A. Randomized, comparative trial on the sustained efficacy of topical imiquimod 5% cream versus conventional ablative methods in external anogenital warts. Eur J Dermatol. 2006;16(6):642–8.
- Geretti AM, Brook G, Cameron C, Chadwick D, French N, Heyderman RS, et al. British HIV Association guidelines on the use of vaccines in HIV-positive adults 2015. https://www.bhiva.org/vaccination-guidelines.aspx. Accessed 14 Dec 2017.
Comments
Vivtenko Lidiya Andreevna - 12.26.2018 - 14:22:11
Of the 12 cases of proven prostate cancer, 100 percent had oncogenic strains of HPV
To post comments you must log in or register
Warts on intimate places: diagnosis
It is impossible to make an accurate diagnosis only with an external examination.
To correctly determine the form of the disease, the doctor will need to conduct laboratory and instrumental studies.
Only a venereologist can reliably establish a diagnosis based on clinical manifestations.
If it is necessary to diagnose formations on the cervix/endourethral, instrumental methods (colposcopy, urethroscopy) are used.
In a number of situations, additional laboratory testing may be required (using PCR methods, histo-, cytological studies).
For laboratory tests, tests of blood and affected tissues are used.
A detailed diagnosis will help the doctor prescribe the optimal treatment.
Itching of the anogenital area
TO
skin itch is a dermatological disease characterized by an unbearable desire to scratch areas of the skin.
Itching, like tactile, temperature and pain sensitivity, is perceived by the same receptors - the endings of unmyelinated nerve fibers located in the subepidermal zone. Next, the impulse is transmitted along the lateral spinothalamic tract to the thalamus and the sensitive zone of the cerebral cortex.
Itching can occur with light touch, temperature changes, emotional stress, or chemical exposure. The pathogenesis of itching has not been fully studied. It has been shown that during itching, mediators are released, including histamine, kinins, enkephalins, pentapeptides, which excite opiate receptors in the brain. Functional disorders of the relationship between excitation and inhibition processes in the cerebral cortex, diencephalic disorders, autonomic dysfunctions, changes in regulatory enzymatic processes are important factors in the pathogenesis of skin itching. The products of cyclooxygenase oxidation of arachidonic acid, in particular cyclic endoperoxide, prostaglandin E, suppress the feeling of itching.
Itching can be limited or generalized, primary or secondary. There are no primary morphological elements for itching. With prolonged itching, lichenification and individual shiny papular elements occur. In the early stages of the disease, there are excoriations covered with hemorrhagic crusts; secondary purulent infection, maceration of the epidermis, and eczematization of the affected areas may occur. With anogenital localization of itching, scratching is radial in nature or is directed from the genitals to the pubis or to the anus.
Itching is paroxysmal in nature, attacks occur more often in the evening or at night, but can also occur during the day. Patients complain of sleep disturbances, insomnia, loss of appetite, bad mood, irritability, lack of interest in the environment, neurotic reactions, and emaciation.
Causes of itchy skin
Anal and genital itching are local forms of itching; in most patients, it spreads to both areas and is diagnosed as anogenital itching.
Anal itching can be primary, occurring without connection with other diseases, or secondary.
The leading role in the pathogenesis of primary anal itching is played by skin contamination with feces due to poor personal hygiene or weakness of the anal sphincter (leakage of feces during the passage of gases and during rectal distension), as well as due to vegetative neurosis with a local increase in sweating. This leads to maceration, skin irritation and itching. Subsequently, lichenification, eczematization, and secondary infection develop, which, naturally, is accompanied by increased itching. A vicious circle arises: itching – scratching – lichenification – increased itching.
Secondary itching can be a symptom of a wide variety of diseases and pathological conditions, including skin ones. Thus, skin itching is one of the characteristic symptoms of lichen planus, scabies, pediculosis, seborrheic eczema, various allergic dermatitis, psoriasis in the progressive stage, eczema in the acute phase, mycotic (especially candidal) skin lesions.
Itching of the anogenital area, as a rule, accompanies parasitic diseases: taeniosis, ascariasis, opisthorchiasis, amebiasis, giardiasis, etc. Sometimes gonorrheal-trichomonas infection is accompanied by skin itching.
Diseases of the stomach (hypo- and hyperacid gastritis, peptic ulcer, polyposis), intestines (colitis, dyskinesia, dysbacteriosis), hemorrhoids, anal fissures, fistulas with macerating discharge, anogenital warts, genital warts can also be accompanied by anogenital itching. Various endocrinopathies (diabetes mellitus, hypo- and hyperfunction of the thyroid gland, etc.), pathology of the pelvic organs (adnexitis, cervical erosion, carurosis of the vulva, prostatitis, urethritis, cystitis, etc.), kidney stone disease, chronic renal failure, various pathology of the liver and biliary tract, oncological pathology are often accompanied by itchy skin.
Skin itching can accompany chronic intoxication (drug addiction, alcoholism, coffee abuse, etc.), various allergic reactions, including to medications, mental illnesses (pathomimia, dermatozoal delirium, psychoses, neuroses, etc.).
Treatment of itchy skin
Treatment of skin itching, including anogenital localization, should be comprehensive. First of all, it is necessary to eliminate or weaken the impact of identified etiological and pathogenetic factors (treatment of scabies, pediculosis, amebiasis, helminthic infestation, trichomoniasis, endocrine, hepatic, renal and other pathologies).
In cases of severe inflammation, glucocorticoids
(prednisolone 10–40 mg per day followed by gradual withdrawal of the drug).
Antihistamines
It is preferable to use in the afternoon, preferably in injection form. Calcium supplements are indicated in case of predominant tone of the parasympathetic system (bright, quickly appearing red dermographism). If the tone of the sympathetic system predominates, calcium supplements should be prescribed with caution due to possible complications from the cardiovascular system.
From external means
corticosteroid ointments, cooling aqueous-alcoholic solutions with menthol, anesthesin, novocaine, lidocaine are used.
Gepatrombin G has proven itself well
, which quite quickly alleviates the condition of patients. Hepatrombin G is a combination drug containing heparin, prednisolone and polidocanol. Heparin has an anticoagulant and anti-inflammatory effect, prednisolone reduces inflammation (including allergic) and itching, polidocanol has an analgesic effect. Indications for the use of Gepatrombin G, along with anal itching, are hemorrhoids, fistulas and anal fissures. Gepatrombin G is available in the form of suppositories and ointments. Suppositories are administered into the rectum 1-2 times a day, and the ointment is used 2-4 times a day (after the exacerbation subsides - 1-2 times a day). The tube with ointment comes with a special tip for inserting it into the rectum. The drug is contraindicated for hemostasis disorders, specific (fungal, mycobacterial) lesions of the anogenital area, in the first trimester of pregnancy.
Depending on the severity of neurotic reactions, sedatives
, tranquilizers (valocordin, corvalol, motherwort tincture, valerian, oxazepam, etc.).
It may be useful to include physiotherapeutic methods in the complex of therapy (inductothermy of the adrenal gland region, acupuncture, electrosleep, infrared laser).
An important point in the treatment of such patients is maintaining personal hygiene (washing the anus, powder with zeolites, talc), and avoiding contact with synthetics.
Intimate warts: how to treat and who to contact?
To properly treat the infection and its consequences, you need to consult a dermatologist-venereologist.
A specialist will be able to accurately determine the course of the disease and select the optimal treatment.
If consultations with other specialists are necessary, they will be prescribed by the attending physician.
The compulsory health insurance system allows you to seek help from state medical institutions.
You can wait several weeks for an appointment at such institutions.
Paid services are available to the patient much faster and allow you to choose a convenient time to visit the doctor.
Warts in intimate places: treatment
How to treat warts in intimate places?
The main method of treating such formations is their removal.
However, when deciding how to get rid of a wart in an intimate place, it is worth understanding that anogenital warts very rarely degenerate into malignant formations.
Sometimes they disappear spontaneously (from a couple of months to two years).
Removing warts in intimate places does not completely eliminate the virus from the human body and does not prevent possible relapses.
For different types of condylomas, different methods of therapy are recommended, the choice of which is the prerogative of the attending physician.
Such techniques include:
- cryotherapy is a method based on the low-temperature effect of liquid nitrogen, does not require pain relief, is well tolerated, and practically does not lead to scar formation
- surgical removal
- intralesional administration of interferons
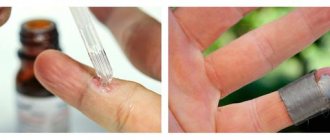
- use of podophyllin, imiquimod, synecatechins (in the form of ointments, creams, solutions)
It can be carried out by the patient independently at home after detailed instructions from the attending physician.;
- the use of solutions of trichloro- and dichloroacetic acids
- combined
Only a doctor can select the optimal treatment option (for example, for internal condylomas, the use of a number of drugs used to remove external ones is prohibited).
Do not forget about the risk of malignancy of formations.
Therefore, you cannot refuse the histological examination recommended by your doctor.
It will help to accurately determine whether there are malignant cells in the condyloma.
To understand how to treat warts in intimate places, it is important to remember about therapeutic interventions.
The proliferation of condylomas is often a consequence of decreased immunity.
Therefore, the doctor may prescribe medications to increase it.
Such medications should be taken strictly as prescribed and in the dosage recommended by the doctor.
Antiviral drugs are used to directly combat the pathogen.
When used correctly, the amount of human papillomaviruses in the blood decreases.
This minimizes the risk of skin tags forming again.
Causes of itching and burning in the anogenital area
The causes of discomfort in the vulva can be provoked by both gynecological pathology and other factors.
Gynecological pathology:
- infection with fungi of the genus Candida (thrush);
- specific vulvovaginitis (gonorrhea, trichomoniasis);
- sexually transmitted infections (chlamydia, mycoplasma);
- vaginal dysbiosis or gardnerellosis;
- pregnancy;
- genital herpes;
- abortions and curettage;
- atrophic vulvovaginitis.
Perhaps itching and burning are caused by the products you use for intimate hygiene. Without identifying the causes and eliminating them, ulcers may develop in the genital area, which are quite difficult to treat. Make an appointment with a doctor and get your life back without discomfort.
Other reasons:
- allergic reactions (to detergents, fragrances, candles and lubricants);
- hypothyroidism, diabetes mellitus;
- psycho-emotional stress (stress, neuroses, depression);
- helminthiases (usually pinworms);
- violation of hygiene;
- trauma to the vulva (rough coitus, ruptures during childbirth, wearing tight synthetic underwear);
- treatment of oncological diseases (chemo- and radiation therapy);
- diseases of the digestive tract;
- treatment with antibiotics and hormones.
Itching and burning in the genital area are always signs of the development of the disease. Even if the problem disappears on its own, this does not mean that you have recovered. Unpleasant symptoms will return, and the disease will become more severe. If you have ever experienced itching and burning in the genital area, immediately make an appointment with your doctor to find out the cause of the discomfort.