Our expert, endocrinologist-nutritionist, creator of the author’s nutrition program, Vadim Krylov, tells the story.
Vitamin B₁ is the most important player in the functioning of the brain and the entire nervous system. It can also be called the “Minister of Energy”, since it is part of several enzymes that play a key role in energy metabolism in the cell. And when there is not enough of it, enzymes stop working and a lack of energy appears. Research shows that B₁ deficiency develops quite quickly and can lead to very negative consequences in the body, and in severe cases, death.
Article on the topic Paradoxical biotin. What is vitamin B7 and why is it so important?
Emperors disease
A condition associated with severe deficiency of vitamin B₁ is called beriberi, or Chinese emperors' disease. The fact is that aristocrats in China, Japan and other countries in this region ate only white refined rice, which was considered noble. But when rice was peeled from the grain shells, useful substances were lost, including vitamin B₁. Due to a very monotonous diet, there were no other sources of thiamine in these countries, which led to its severe deficiency.
Vitamin B₁ is water soluble. It does not accumulate in the body, and excess is excreted in the urine. This feature is typical of all B vitamins. Therefore, they must be consumed every day to avoid deficiency. It is important that there is no overdose of vitamin B₁, even if you consume it many times more than the recommended daily intake. The body will take everything it needs, and the rest will be excreted through the kidneys.
First of all, thiamine is needed for the synthesis of acetylcholine, one of the most important neurotransmitters of the nervous system, on which the conduction of signals to the brain and nerves depends. Therefore, with a lack of thiamine, the nervous system and psyche are primarily affected: weakness, fatigue, and memory deterioration occur. In severe deficiency, as in beriberi, nerve conduction is disrupted, paralysis of the legs occurs, people stop walking, and death can occur.
Click to enlarge
Vitamin B1
Thiamine (vitamin B1) is an essential nutrient that serves as a cofactor for a number of enzymes, mainly with mitochondrial localization. Some thiamine-dependent enzymes are involved in energy metabolism and nucleic acid biosynthesis, while others are part of the antioxidant mechanism. The brain is very vulnerable to thiamine deficiency due to its dependence on mitochondrial ATP production. This situation is more obvious during the rapid growth of children, in which thiamine deficiency is usually associated with malnutrition or genetic defects. Thiamine deficiency contributes to a range of neurological and psychopathological symptoms (confusion, memory loss and sleep disturbances) to severe encephalopathy, ataxia, congestive heart failure, muscle atrophy and even death.
The primary nutrient thiamine (vitamin B1) is a water-soluble sulfur-containing vitamin belonging to the B vitamin complex. Not being endogenously synthesized, the only available source of thiamine is from certain foods (beef, poultry, grains, nuts and beans). The body does not store thiamine >30 mg, and the half-life for thiamine is only 9–18 days. With an average food intake of 2000 kcal (consumed daily), the minimum requirement for thiamine is 0.66 mg, however, the recommended daily intake for adult men and women is 1.2 and 1.1 mg, respectively. During pregnancy or breastfeeding, the requirement for vitamin B1 increases to 1.4 mg/day. In children, the recommended dietary allowance (RDA) varies by age and ranges from 0.2 mg (birth to 6 months) to 0.6 mg (6 months to 8 years). In the human body, tissues rich in thiamine include skeletal muscle, heart, liver, kidneys and brain.
In developed countries, the predominant use of industrial food processing often depletes thiamine along with other vitamins and nutrients. Increased consumption of processed foods in the form of simple carbohydrates not supplemented with adequate levels of thiamine has been termed "caloric malnutrition." On the other hand, at least 29% of obese patients undergoing bariatric surgery are thiamine deficient. Because thiamine is a key factor in glucose metabolism, increasing carbohydrate intake will proportionally increase dietary thiamine requirements (minimum 0.33 mg per 1000 kcal). So, rather than focusing on the RDA of thiamine, it is important to compare your intake to your carbohydrate intake as well as your total calorie intake.
In developing countries, thiamine deficiency remains a widespread problem due to high consumption of white rice. As home milling methods are replaced by industrial rice milling and processing, important nutrients (such as thiamine) in the bran are removed. Asian countries consume about 90% of the world's rice, supplying, according to experts, 60% of the population's daily dietary energy requirements, and, therefore, thiamine deficiency is quite common among 15% of the adolescent population. Thiamine deficiency can develop from consuming diets contaminated with thiamine-metabolizing enzymes (eg, thiaminase) or that have undergone thiamine inactivation by heat and/or sulfur dioxide. Excessive consumption of tannin-containing foods or foods rich in caffeine, theobromine, and theophylline (such as those found in coffee, chocolate, and tea, respectively) can inactivate thiamine, thereby interfering with its status.
Other risk factors that increase the likelihood of insufficient thiamine intake include aging, low economic status, eating disorders, medical illnesses particularly affecting the gastrointestinal tract, artificial nutrition, bariatric surgery, diabetes and alcohol abuse. Unmet needs for increased dietary thiamine intake have been reported during lactation, pregnancy, and increased physical activity. During lactation, children have an increased risk of developing vitamin deficiency, in particular, thiamine deficiency in mothers. For example, 27% of women of childbearing age in Cambodia have thiamine deficiency, and 38% of infants are diagnosed with thiamine deficiency, a critical problem that contributes significantly to mortality in 3-month-old infants. However, even with adequate thiamine intake, thiamine deficiency may be due to genetic factors , that is, pathogenic gene mutations in key regulators of the thiamine transformation pathway, including thiamine pyrophosphokinase 1 (TPK1), thiamine diphosphate kinase (TDPK), thiamine triphosphatase (THTPA), and thiamine transporters (SLC25A19, SLC19A2 / THTR1 and SLC19A3 / THTR Organic cation transporter 1 (OCT1) has also been shown to act as a thiamine transporter in the liver.
Regardless of the underlying cause of thiamine deficiency, most symptoms occur at the neurological level. Thiamine deficiency may cause damage to brain tissue by inhibiting brain energy utilization, given the critical role of thiamine-dependent enzymes associated with glucose utilization. This is supported by the significant uptake of thiamine across the blood-brain barrier, highlighting the brain's high thiamine requirement and supply to maintain adequate brain function, especially in those brain regions that require high levels of thiamine metabolism and turnover.
As with most hydrophilic micronutrients, thiamine absorption occurs primarily in the small intestine. In the digestive tract, food proteins are hydrolyzed, releasing thiamine. In the intestinal lumen, alkaline phosphatases catalyze the hydrolysis of thiamine phosphorylated derivatives into free thiamine. Unphosphorylated free thiamine at concentrations greater than 1 μM enters the enterocyte by passive diffusion, whereas at lower levels it is transported through the thiamine/H+ repletion system (thiamine transporter 1 or THTR1) in an energy-dependent manner. Under conditions of thiamine deficiency, upregulation of thiamine transporter 2 (THTR2) expression was observed in Caco2 cells in culture, suggesting that diet may modulate the expression of this transporter. Inside the enterocyte, thiamine is phosphorylated to thiamine pyrophosphate (TPP) by TPK1. Most of the TPP is then dephosphorylated to thiamine monophosphate (TMP) to cross the enterocyte basement membrane. TMP is released into the bloodstream through an ATPase-dependent transport system. Free thiamine can also enter the blood through the thiamine transporter 2 (THTR2), located primarily on the basolateral membrane of the enterocyte. Once in the blood, while very low levels of TMP and thiamine circulate freely in plasma or serum, more than 90% of phosphorylated thiamine (in the form of TPP) is present in red and white blood cells. Notably, carrier isoform 3 of SLC44A4 was recently described as a carrier of TPP in the colon. SLC44A4 was originally described as a choline transporter associated with non-neuronal choline synthesis and required for the efferent innervation of hair cells in the olivocochlear bundle to maintain the physiological function of the outer hair cells and protect the hair cells. from acoustic trauma. Recent evidence indicates that this vehicle may mediate the absorption of microbiota generated by TPP (especially in infants) and promote host thiamine homeostasis.
Cellular uptake of thiamine from the bloodstream can be mediated by either of two high-affinity carriers: THTR1 (encoded by SLC19A2) and THTR2 (encoded by SLC19A3). These transporters are expressed ubiquitously, but THTR1 is most abundant in the intestine, skeletal muscle, nervous system, and eyes, followed by the placenta, liver, and kidney, while THTR2 is found primarily in adipose tissue, liver, lymphocytes, spleen, gallbladder, and placenta. pancreas and brain. After intracellular transport, free thiamine is rapidly phosphorylated to TPP by thiamine pyrophosphokinase (TPK1). A second kinase, TDPK, adds a phosphate group to TPP to generate thiamine triphosphate (TTP). TPP and TTP can be dephosphorylated, respectively, to TMP and TPP by the phosphatases prostatic acid phosphatase (ACPP) and THTPA, respectively.
Up to 90% of the total thiamine in the body remains in its diphosphate, the metabolically active form (TPP), while the rest is in the form of TMP and TTP. TPP is a cofactor for several thiamine-dependent enzymes involved in carbohydrate and fatty acid metabolism, namely cytosolic transketolase (TKT), peroxisomal 2-hydroxyacyl-CoA lyase 1, and three mitochondrial enzymes (pyruvate dehydrogenase, α-ketoglutarate dehydrogenase, and branched chain enzymes). -chain α-keto acid dehydrogenase complexes. The biochemical role of TPP is well understood, but the biological significance and contribution of TTP is not entirely clear. Previously thought to be a specific neuroactive form of thiamine, TTP (constituting ∼10% of the total brain thiamine pool) has recently been reported to be involved in membrane excitability and nerve conduction by acting as a modulator of sodium chloride channel permeability.
In the cytosol, TPP acts as a cofactor for TKT, a key enzyme in the nonoxidative branch of the pentose phosphate pathway (PPP). This metabolic pathway generates nicotinamide adenine dinucleotide phosphate (NADPH) and ribose 5-phosphate (R5P). NADPH is a key reducing agent in biosynthetic reactions and is one of the substrates of biosynthetic enzymes (fatty acid synthesis) and antioxidant enzymes such as the glutathione peroxidase reductase system and thioredoxin peroxidases. The important involvement of R5P in DNA and RNA biosynthesis highlights the critical role of thiamine in highly proliferative tissues.
Based on its role in biochemical pathways, it is predicted that thiamine deficiency will lead to increased oxidative stress and decreased cell proliferation, as well as decreased fatty acid synthesis (including myelin), with severe consequences, especially during brain development. Consistent with this proposal, thiamine deficiency reduces TKT activity and leads to impaired PPP and decreased neurogenesis in the cortex and hippocampus during neurodevelopment.
Peroxisomes play an important role in the catabolism of hydrogen peroxide, as well as in the shortening of very long fatty acids (which cannot be directly catabolized by mitochondrial β-oxidation) and α-oxidation. In the latter process, the TPP-dependent enzyme 2-hydroxyacyl-CoA lyase 1 (HACL1) catalyzes the breakdown of 3-methylbranched and 2-hydroxy straight-chain long-chain fatty acids. Phytanic acid (a 3-methyl-substituted 20-carbon branched-chain fatty acid), unlike most fatty acids, cannot undergo β-oxidation due to the presence of a methyl group at position 3. As such, it is cleaved by HACL1 as a result of the initial α- oxidation. This branched chain fatty acid is obtained from the diet, especially dairy products and red meat. Impaired phytanic acid catabolism due to inadequate TPP levels leads to triglyceride accumulation, which can cause negative effects such as cerebellar ataxia, peripheral polyneuropathy, vision and hearing loss, anosmia, and in some cases, cardiac dysfunction and epiphyseal dysplasia. Symptoms caused by thiamine deficiency are characteristic of Refsum disease, which is caused by pathogenic mutations in HACL1. Some of the symptoms are also seen in autosomal recessive systemic disorder, Zellweger syndrome, and other peroxisomal diseases, including neonatal adrenoleukodystrophy. Zellweger syndrome is caused by pathogenic mutations in the pexin genes, which encode proteins required for the assembly of functional peroxisomes. It is characterized by a deficiency in the peroxisomal fatty acid oxidation pathway, causing severe neurological and hepatic dysfunction, as well as craniofacial disorders.
The majority (∼90%) of cytosolic TPP is transported into mitochondria by the mitochondrial thiamine pyrophosphate transporter MTPPT, the product of the SLC25A19 gene. This transporter mediates the exchange of cytosolic TPP for mitochondrial TMP; Once in the cytosol, TMP is metabolized and converted back to TPP. In mitochondria, TPP is a critical cofactor for three enzymes, namely pyruvate dehydrogenase, α-ketoglutarate dehydrogenase, and branched-chain α-ketoacid dehydrogenase (PDH, αKGDH, and BCKDH, respectively).
The pyruvate dehydrogenase complex is a multisubunit complex that catalyzes the TPP-dependent decarboxylation of pyruvate, generating acetyl-CoA, which then enters the Krebs cycle. Regulation of PDH activity represents a key metabolic “switch” influencing the choice of “fuel”, that is, between fatty acid oxidation and glycolytic flux. It has been suggested that failure to regulate fuel selection for metabolic energy production underlies "metabolic rigidity" leading to metabolic disorders. Therefore, thiamine-mediated inhibition of the PDH complex blocks the system from oxidizing glucose to pyruvate, resulting in increased lactate and decreased cellular ATP production. As expected, in severe cases, metabolic deficiency manifests as fatal lactic acidosis in newborns, while in milder cases, neurological conditions can lead to structural abnormalities in the central nervous system (CNS), seizures, mental retardation and spasticity.
In cases of thiamine deficiency, the most affected areas of the brain appear to be the cerebellum, mamillary bodies, thalamus, hypothalamus, and brainstem in adults. Regarding thiamine deficiency, Zhao et al. (2009) showed that in mice, thiamine deprivation for 14 days resulted in varying degrees of enzyme deficiency when tested for TKT, PDH, and αKGDH activity in the cortex and hippocampus.
Pathogenic mutations in genes encoding enzymes and transporters involved in thiamine metabolism result in symptoms similar to those found in diet-based thiamine deficiency and overlap with mitochondrial dysfunction disorders. These mutations, affecting the genes responsible for thiamine transporters 1 (SLC19A2; OMIM 249270) and 2 (SLC19A3; OMIM 607483), constitute the main cause of suboptimal absorption of thiamine in the intestine and, as a consequence, insufficient cellular distribution of thiamine throughout the body.
As stated above, the pathology of thiamine deficiency involves impaired mitochondrial energy production in the form of ATP when using pyruvate-generating substrates (eg glucose), as well as increased oxidative stress. Under these conditions, glucose forms pyruvate through glycolysis, which cannot enter the Krebs cycle in the form of acetyl-CoA due to low PDH activity. As such, pyruvate is transaminated to Ala or reduced to lactate by lactate dehydrogenase. This is consistent with the increased levels of lactate and organic acids observed in CSF, urine, and blood in thiamine deficiency.
The human central nervous system has a high energy requirement: 2% of body weight controls about 20% of total metabolic expenditure, most of which is spent on excitatory action potentials, on the transmission of signals between neurons, through chemical synapses, and axonal growth. and myelination. Since glucose is the primary fuel for energy production in the brain, it is not surprising that mitochondrial dysfunction and subsequent impairment of glucose metabolism are associated with several neurological and neurodevelopmental disorders and major mental illnesses such as depression and schizophrenia.
The neurological symptoms of thiamine deficiency are similar to the defects that most commonly manifest as Li-Lee syndrome involving the basal ganglia. Therefore, the nervous system, which is specialized to use glucose for energy, appears to be most vulnerable to PDHC deficiency due to TPP depletion. In the brain, poor mitochondrial ATP production will limit the maintenance of membrane potential through the action of Na+,K+-ATPase, thereby impairing nerve conduction and synaptic processes. Additionally, increased oxidative stress due to lower TKT activity will damage critical biomolecules by initiating lipid peroxidation and oxidative damage to proteins, leading to fragmentation, post-translational modifications, and cross-linking. Modification of epitopes on normal, endogenous molecules can lead to activation of microglia and immune cells, exacerbating oxidative stress-induced damage.
Blood and CSF thiamine levels provide limited information when assessing a subject's thiamine status as they do not necessarily reflect the metabolic function of thiamine or a direct relationship to tissue levels. Thus, erythrocyte TKT and, if possible, assessments of other tissue-specific TPP-dependent enzymes ( PDH, αKGDH ) are considered gold standards. Baseline TKT activity is usually expressed in units per gram of hemoglobin (g Hb), but more importantly, the percentage of TKT activation in TPP supplements is calculated (0-15% is considered normal).
TKT activation ratio (red blood cells) and/or TPP-dependent enzyme activity (white blood cells, skin fibroblasts, and muscle biopsies) are usually accompanied by testing of serum lactate and pyruvate levels, BCAAs, organic acids, and brain imaging techniques. The only cases where assessment of free thiamine in plasma/serum and CSF appears to be a valuable diagnostic tool are in cases of pathogenic mutations in SLC19A3. Similarly, urinary excretion of thiamine is also not a reliable method for assessing its level in the body since it depends on its consumption and absorption. It is typically expressed per unit of creatinine to account for renal function, and age should be taken into account as normal values vary between children [120 nmol/mmol creatinine aged 1–13 years] and adults [220 nmol/mmol creatinine aged > 18 years old].
Unfortunately, the early symptoms of thiamine deficiency are subtle or not distinct enough to make a direct diagnosis. These include loss of appetite, nausea, weakness, apathy, fatigue, irritation, sleep disturbances, anorexia and abdominal discomfort. Additionally, identifying specific clinical symptoms of thiamine deficiency is problematic because it is obscured by the influence of other comorbid conditions such as infections and/or various nutritional disorders.
The clinical classification of thiamine deficiency is usually divided into "dry" (or neurotic, characterized by polyneuropathy, decreased knee-jerk and other tendon reflexes, and progressive severe muscle weakness) and "wet" (or cardiac, characterized by swelling of the legs, body and face, high cardiac output, ventricular insufficiency and congestion in the lungs).
If generalized thiamine deficiency is suspected early, prompt administration of thiamine is recommended and usually effective treatment. The literature reports a wide range of therapeutic approaches and doses of thiamine from 1.5 to 600 mg/day, with 10–20 mg/day in divided doses over several weeks for mild polyneuropathy and 20–30 mg/day. moderate to severe, usually until symptoms disappear. Typically, thiamine deficiency is treated with doses of 5–30 mg/day intravenously (IV) or intramuscularly (IM) three times daily, then 5–30 mg/day orally until symptoms resolve. However, this approach is markedly less effective for people with chronic forms of thiamine deficiency disorders, including encephalopathies or TPK1 deficiencies. In the latter case, it would be worthwhile to investigate treatment directly with TPP; however, it is unclear whether this form of phosphorylated thiamine will cross the blood-brain barrier and/or reach subcellular targets such as PDH.
A number of studies have shown an inverse association between thiamine levels and depressive symptoms in adults. The study found that depressive symptoms significantly improved in patients with major depression after 6 weeks of taking thiamine compared to placebo. The effects of thiamine may be significant as a palliative treatment for postpartum depression and play an important role in the child's subsequent cognitive development. PPD is associated with an increased risk of learning disabilities, attention-deficit/hyperactivity disorder (ADHD), and anxiety disorders in young children, making PPD a critical problem for both mother and infant. Therefore, thiamine supplementation may improve carbohydrate metabolism, mitochondrial function, and energy production in the brain to some extent.
Alcohol and vitamin B1
The last major outbreak of beriberi was 65 years ago in the Philippines. Now they have learned to prevent this disease, so it does not occur even in those countries where rice still remains the main food. However, a peculiar analogue of this disease is often found even in highly developed countries. It's called Wernicke-Korsakoff syndrome, a brain disorder caused by thiamine deficiency. For alcohol lovers who drink a lot and constantly, a deficiency of vitamin B₁ leads to damage to the nerves, the legs begin to tremble and do not obey well, then the person stops walking altogether. The fact is that when drinking alcohol, the need for vitamin B₁ increases significantly, and its supply decreases due to poor nutrition, typical of alcoholics.
A similar situation happens with those with a sweet tooth. A large amount of carbohydrates in food requires an increased amount of thiamine, but sweets and baked goods, which are usually made from refined wheat flour, do not have this vitamin. It is present in the shells of grains, and if they are removed, vitamin B₁ is lost. Therefore, choose bread only from whole grain flour, and choose unpolished porridges that have retained their grain shells.
May there always be sunshine! Why do you need vitamin D Read more
Forecast and preventive measures
Timely treatment of hypovitaminosis allows one to count on a complete recovery of the patient. A favorable prognosis is formed with moderate vitamin deficiency and normalization of the diet of a child or adult. Severe forms of vitamin deficiencies do not always allow the normal functioning of individual body systems to be restored. If left untreated, vitamin deficiency can be fatal.
Prevention of pathology is based on a balanced diet. Signs of vitamin deficiency will not appear in children and adults if they systematically consume a sufficient amount of vegetables, fruits, and herbs. In autumn and winter, you should include freshly squeezed juices, citrus fruits and sauerkraut in your diet.
Deficiency Symptoms
At first there are no specific symptoms. Weakness, fatigue, shortness of breath and palpitations during physical activity - all this happens with a host of other diseases. How can we determine what caused these symptoms: a lack of vitamin or something else? First, think about whether there are real reasons for such problems. Secondly, analyze your diet - does it contain enough foods with vitamin B₁ (see table). If there is no reason, and the composition of the products is not ideal, most likely the problem is in the vitamin.
Article on the topic
We treat anemia. Why is vitamin B12 deficiency dangerous?
Treatment of B vitamin deficiency
There are two types of correction of B vitamin deficiency: therapeutic and preventive.
Therapeutic tactics involve the use of high doses of B vitamins over long courses. The correction is carried out under the strict supervision of a doctor in the presence of clinical and laboratory data confirming vitamin deficiency. Currently, primary nutritional deficiencies are rare. Basically, this form of deficiency of B vitamins develops against the background of pathology of the gastrointestinal tract or with hereditary disorders of vitamin metabolism. In this case, taking oral forms of drugs does not always have the expected therapeutic effect, so some groups of patients are prescribed injections of multi- or single-vitamin preparations.
Preventive tactics for eliminating deficiency of B vitamins involve taking medications in a dosage close to the daily requirement. Such therapy can be prescribed without proven vitamin deficiency.
Medical genetics diagnoses hereditary and acquired disorders of vitamin metabolism, which allows you to select tactics for correcting hypo- and avitaminosis.
Important
Requirement for vitamin B1 (mg) Children
- up to 12 months – from 0.3 to 0.5
- 1–3 years – 0.8
- 3–7 years – 0.9
- 7–11 years – 1.1
- 11–14 years old – 1.3
- 14–18 years old:
- boys – 1.5,
- girls – 1.3
For adults
- Men and women – 1.5
- Pregnant women – 1.7
- Nursing mothers – 1.8
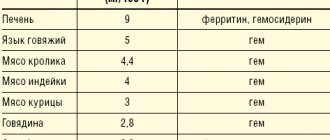
Where is there a lot of thiamine?
| Products | Vitamin B content per 100 g | Percentage of DNP for adults (1.5 mg/day) |
| Sunflower seeds. | 1.5–1.8 mg | 123% |
| Sesame seeds. | 1.27 mg | 85% |
| Lean pork; high-quality sausages without additives: Braung Swiss, servelat; Red caviar; peanut. | 0.5–0.55 mg | 35–37% |
| Oatmeal: “Hercules” and others; beans; lentils; cashew nuts and hazelnuts. | 0.45–0.5 mg | 30–33% |
| Pork is fatty; buckwheat; millet; low grade wheat and rye flour, wallpaper, whole grain; walnuts and pine nuts. | 0.37–0.44 mg | 25–29% |
| Bread made from whole grain or wallpaper flour, with cereals; Borodinsky from 1st grade flour, but not the highest; green pea; high-quality boiled sausages without additives: doctoral, amateur, dairy, Russian; beef liver; some types of fish: pink salmon, salmon, chum salmon; squid. | 0.16–0.35 mg | 11–25% |