Iron in the blood: general information
All inorganic substances in the human body are divided into macro- and microelements. The former include calcium, sodium, potassium and magnesium. The prefix “macro” indicates a relatively large content of these elements in the body.
Important!
Iron is not produced in the body and comes only from food. The most iron is found in red meat, mushrooms, cocoa, legumes, and some vegetables and fruits.
Iron is a micronutrient. But despite its small amount, this element plays a vital role in maintaining the vital functions of the body. Lack or excess of iron negatively affects health, which is expressed by a number of symptoms and diseases.
The main functions of iron include:
- Oxygen transport. Iron is a component of the hemoglobin pigment found in red blood cells (erythrocytes)1. Thanks to iron, hemoglobin is able to carry oxygen from the alveoli of the lungs to other organs and tissues.
- Hematopoiesis. In the bone marrow, iron takes part in hematopoiesis - the formation of blood cells, in particular, red blood cells.
- Detoxification of the body. Iron is part of a number of liver enzymes that take part in the destruction of toxic substances.
- Immunity. The level of leukocytes, as well as their activity, also depends on the level of iron.
- Cell division. Iron is part of the enzymes involved in DNA synthesis, a process necessary for normal cell division.
- Hormone synthesis. With the participation of iron, the synthesis of thyroid hormones occurs.
Iron contained in the human body comes in several types:
- Functional. This is iron, which is directly involved in many life processes. For example, iron contained in hemoglobin ensures the transport of oxygen from the lungs to each cell. In addition, this trace element is contained in myoglobin and some vital enzymes.
- Transport. This is iron that circulates in the blood. Note that there is no free iron in the blood (since it is toxic to tissues). Iron is transported in combination with carrier proteins. These are transferrin, lactoferrin and mobileferrin1.
- Deposited. The human body also has some iron reserves. Iron is mainly stored in the liver and spleen in the form of ferritin. It is a complex water-soluble protein complex. To a lesser extent, iron is deposited in the form of another protein - hemosiderin, which is formed during the destruction of hemoglobin.
- Free. Iron is found in this form inside cells. Due to its toxicity, free iron does not move inside the cell independently, but with the help of the protein mobilferrin.
About 75% of all iron in the human body is heme. It is found in hemoglobin, myoglobin and some enzymes. The rest, non-heme iron, is enclosed in transport and storage proteins.
The functions of iron in the body and signs of its deficiency. Image: MedPortal.
Iron as a vital nutrient
The article describes the role of iron in the human body. Conditions leading to iron deficiency are considered. Recommendations for therapy with iron supplements are given.
It has been shown that in most cases, the use of vitamin and mineral complexes containing iron is sufficient to correct iron deficiency.
Rice. 1. Chemical formula of a hemoglobin molecule containing an iron atom
Rice. 2. Receipt of exogenous iron into tissues
Biological role of iron
Iron in the form of various compounds is very common in nature, including in many foods. Iron ions participate in the following chemical reactions: transport of electrons, cytochromes, iron sulfur proteins; transport and storage of oxygen with myoglobin and hemoglobin; formation of active centers of redox enzymes - oxidases, hydroxylases, SOD (Fig. 1).
The physiological costs of iron are relatively small; with acute or chronic blood loss, the need for iron increases. Iron is necessary for normal erythropoiesis and enters the bone marrow in the following ways: during the destruction of red blood cells, from the depot, with food and water. For normal erythropoiesis, the daily diet of an adult requires 12–15 mg of iron. Iron is deposited in various organs and tissues, mainly in the liver and spleen.
In the human body, iron is part of almost 70 essential enzymes. The total iron content in the body is about 4.5–5 g, of which 75–80% is iron in hemoglobin, which ensures the transport of oxygen to tissues; 5–10% is part of myoglobin; 1% – in the composition of respiratory enzymes that catalyze respiration processes in cells and tissues. 20–25% of the iron contained in the body is reserve. Physiological losses of iron in urine, sweat, feces, hair, and nails, regardless of age and gender, are about 1 mg/day. In women, during normal menstruation, which lasts 3–4 days, iron loss is about 15 mg (30–50 ml of blood). With hyperpolymenorrhea (up to 50–250 ml of blood), iron loss increases significantly. During pregnancy, childbirth and lactation, up to 1700–1800 mg of iron is lost. Due to the breakdown of hemoglobin, about 21–24 g of iron are released per day, which is many times higher than the intake of iron from the digestive tract (1–2 mg/day) [1]. Thus, with an increased need for an element, its deficit begins to be replenished at the expense of reserve and then transport funds.
In celiac disease (celiac disease), iron and B12 deficiency anemia is detected in 1.8–14.6% of cases, usually in combination with malabsorption of other nutrients [2].
Regulation of iron homeostasis
It should be noted that the amount of iron in the body is relatively stable and is provided by the intake of iron from food and through iron recycling, starting with the lysis of old red blood cells. This process involves macrophages of the spleen, red bone marrow and, to a lesser extent, Kupffer cells. Hydrochloric acid in gastric juice, ascorbic acid in food, and the enzyme ferroreductase promote the absorption of iron in the intestine, converting ferric iron into ferrous iron. A number of enterocyte receptors facilitate the passage of iron into the cell and from there into the plasma. The mucous membrane of the small intestine contains the iron transport protein transferrin. In the cell, the transferrin-iron complex breaks down, and the iron binds with another transport protein, ferritin. Precursor cells of mature red blood cells accumulate iron in ferritin. It is subsequently used when the cell begins to synthesize large amounts of hemoglobin.
Iron is stored in the form of ferritin (an easily mobilized form of reserve) or hemosiderin (a difficult to mobilize form of reserve). Plasma transport includes transferritin iron and accounts for approximately 1% of total body iron.
Iron, which comes from enterocytes (5%) and is released due to the recycling of old red blood cells with the participation of mononuclear macrophages (95%), is transferred mainly to the bone marrow. With an excess intake of iron, its special biochemical form is formed that can generate free radicals, which leads to complications. Excessive amounts of iron in food or drug therapy over several days can lead to decreased iron absorption. This phenomenon, called a “mucus block,” can occur even when the body is depleted of iron.
Excess iron can be observed in hematological patients after repeated blood or red blood cell transfusions. In such cases, the use of iron chelators is indicated, for example, drugs such as deferoxamine and deferasirox [3]. A similar overload of iron supplements is observed, for example, in myelodysplastic syndrome due to repeated blood transfusions. In these cases, chelation therapy is also indicated [4]. The possibility of accelerated development of osteoporosis with iron overload was also confirmed in an experiment on mice [5].
The following protein compounds take part in the regulation of iron homeostasis.
Hepcidin is an antimicrobial peptide synthesized in the liver in the form of a prepropeptide of 84 amino acids, excreted into the bloodstream in the form of a mature structured peptide of 25 amino acids in the presence of 8 cysteine molecules forming four disulfur bridges. With an increase in the amount of hepcidin, iron absorption decreases and its retention in the macrophage system increases. Conversely, reduced hepcidin production improves iron absorption and reduces iron retention in macrophages. Thus, hepcidin regulates the absorption of iron and its metabolism, protecting the body from excess iron. The experiment established that hepcidin, by reducing iron toxicity, helps reduce the area of infection of the heart muscle during myocardial infarction [6]. Iron deficiency anemia is often detected in renal failure. In some patients, it is refractory to therapy with iron preparations, erythropoietin and is considered as iron-refractory iron deficiency anemia. It is assumed that the described pathology is genetically determined and associated with disturbances in the metabolism of hepcidin, which plays an important role in iron homeostasis [7].
The 343 amino acid HFE protein plays an indirect role in iron metabolism. The absence of HFE protein on the cell surface leads to hyperabsorption and iron overload, the mechanisms of this process have not yet been studied. The relationship between hepcidin and the HFE protein currently needs to be clarified.
An important role in the regulation of erythropoiesis belongs to the nuclear factors GATA-1 (intranuclear transcriptional regulator in the erythron) and NFE-2. The absence of GATA-1 prevents the differentiation of erythrocytes, the lack of NFE-2 impairs the absorption of iron in the intestine and the synthesis of globin.
Regulation of iron homeostasis is also under the control of the endocrine glands. For normal erythropoiesis, hormones are needed that regulate the metabolism of proteins (somatotropic hormone of the pituitary gland, thyroid hormone thyroxine, etc.) and calcium (parathyroid hormone, thyrocalcitonin). Male sex hormones (androgens) stimulate erythropoiesis, while female sex hormones (estrogens) inhibit it, which apparently causes a lower number of red blood cells in women compared to men.
It has been established that the key factor that ensures the proliferation and differentiation of erythroid cells and also prevents their apoptosis is the glycoprotein hormone erythropoietin circulating in the blood. Erythropoietins are present in the blood of animals and people experiencing hypoxia, which is observed during anemia, rising to heights, muscular work, etc. It has been established that the kidneys are the site of erythropoietin synthesis; they are also formed in the liver, spleen, and bone marrow. Erythropoietic activity is possessed by polypeptides of erythrocytes, the molecular weight of which does not exceed 10,000. Erythropoietins act directly on the progenitor cells of the erythroid series:
- accelerate the transition of bone marrow stem cells into erythroblasts;
- increase the number of mitoses of erythroid cells; accelerate the maturation of normoblasts and reticulocytes.
For normal erythropoiesis, vitamins are necessary, primarily vitamin B12 and folic acid, which take part in the synthesis of globin. Other B vitamins also play an important role in the regulation of erythropoiesis [8].
Erythropoiesis is influenced by interleukins synthesized by monocytes, macrophages, lymphocytes and other cells. The production of erythropoietin is regulated at the level of transcription of its gene. At a normal level of tissue oxygenation, the concentration of erythropoietin, as well as the volume of circulating red blood cells, remains constant. Thus, hypoxia is the only physiological stimulus that increases the number of cells synthesizing erythropoietin.
Iron-containing organic compounds
Iron in the body is divided into cellular and extracellular.
Cellular iron
1.
Hemoproteins, the main structural element of which is heme (hemoglobin, myoglobin, cytochromes, catalase and peroxidase). In this case, hemoglobin carries exogenous oxygen and endogenous carbon dioxide. The red blood cell in this case is a kind of buffer system that regulates the overall gas transport function.
2.
Iron-containing enzymes of the non-heme group (succinate dehydrogenase, acetyl-coenzyme A dehydrogenase, NADH-cytochrome, C-reductase, etc.). In them, iron is not included in the heme group and is necessary only for transfer reactions. Iron-containing enzymes and non-heme iron are found primarily in mitochondria. Mitochondrial iron is necessary for tissue differentiation processes, and extramitochondrial iron plays an important role in the processes of cell growth and respiration. The most studied and important enzymes for the body are cytochromes, catalase and peroxidase. The main biological role of most cytochromes is participation in electron transfer in the processes of terminal oxidation in tissues.
Cytochromes are divided into the following groups:
- A – cytochromes with a heme group connecting formylporphine;
- B – cytochromes with protoheme group;
- C – cytochromes with a substituted mesoheme group;
- D – cytochromes with a heme group connecting dehydroporphin.
The human body contains the following cytochromes: a1, a3, b, b5, c, c1, P450. They are lipid complexes of hemoproteins and are tightly bound to the mitochondrial membrane. Cytochromes B5 and P450 are found in the endoplasmic reticulum.
Cytochrome oxidase is the final step in mitochondrial electron transport, responsible for the formation of ATP during oxidative phospholation in mitochondria.
Catalase is one of the most important enzymes that protects red blood cells from oxidative hemolysis. Like cytochrome oxidase, the enzyme consists of a single polypeptide chain connected to a heme group.
Peroxidase is found mainly in leukocytes and in the mucous membrane of the small intestine in humans. It plays a protective role, protecting cells from destruction by peroxide compounds.
Myeloperoxidase is an iron-containing heme enzyme found in azurophilic granules of neutrophil leukocytes and released into phagocytic vacuoles during granule lysis. The destruction of the bacterial cell wall protein activated by this enzyme is fatal to the microorganism.
3.
Ferritin and hemosiderin of internal organs. Ferritin and hemosiderin are found mainly in the reticuloendothelial system of the liver, spleen and bone marrow. Approximately one third of the human body's reserve iron, primarily in the form of ferritin, is found in the liver. Iron reserves, if necessary, can be mobilized for the body's needs. There is no doubt that the concentration of serum ferritin reflects the state of the iron reserve fund in the human body.
Hemosiderin is the second reserve iron compound in the cell and contains significantly more iron than ferritin. Unlike ferritin, it is insoluble in water.
4.
Iron, loosely bound to proteins and other organic substances.
Extracellular iron
Transferrin is a specialized iron-binding protein that is found in small amounts in blood plasma. Transferrin not only transports iron to various tissues and organs, but also recognizes hemoglobin-synthesizing reticulocytes and other iron-requiring cells. Transferrin transfers iron to them only if the cells have specific iron receptors. When receptors are lost, the cell loses its ability to utilize iron. Thus, transferrin functions as a transport vehicle for iron, the metabolism of which in the human body depends both on the total supply of iron in the blood plasma and on its amount captured by various tissues according to the number of specific receptors for iron in these tissues. The most important reason determining the level of plasma iron is the interaction of the processes of synthesis and breakdown of red blood cells. The amount of iron bound to transferrin is about 0.1% (4 mg) of total iron in the body. The total iron-binding capacity of plasma, determined by the concentration of transferrin in it, ranges from 44.7 to 71.6 µmol/l, and the free iron-binding capacity, or reserve capacity of transferrin, is 28.8–50.4 µmol/l in a healthy person.
In the plasma of a healthy person, transferrin can be found in 4 molecular forms:
- apotransferrin;
- monoferric transferrin A – iron occupies only the A-space;
- monoferric transferrin B – iron occupies only the B-space;
- Diferric transferrin – A- and B-spaces are occupied.
Lactoferrin. This protein is found in breast milk, tear fluid, bile, synovial fluid, pancreatic juice and small intestinal secretions. Under physiological conditions, this iron-binding protein is saturated with iron up to 20%. It is found in negligible quantities in blood plasma. Despite the similarity of lactoferrin and transferrin, these iron-binding proteins differ from each other in antigenic properties, composition of amino acids, proteins and carbohydrates. Lactoferrin is involved in immune processes and iron absorption in the small intestine. The advantage of using bovine lactoferrin fortified with iron in pregnant women with anemia compared with the commonly used ferrous sulfate has been shown. Hematological indicators of anemia improved in parallel with an increase in serum prohepcidin levels [9]. Disturbances in iron metabolism can lead to iron overload (hemochromatosis) or, conversely, anemia. A connection has been established between iron metabolism, insulin resistance and obesity [10]. Figure 2 shows a diagram of iron intake into the body [11].
A.P. Avtsyn describes the following scheme of iron metabolism: “In quantitative terms, the metabolic cycle is of greatest importance: plasma → red bone marrow → red blood cells → plasma. In addition, the cycles function: plasma → ferritin, hemosiderin → plasma and plasma → myoglobin, iron-containing enzymes → plasma. All these three cycles are interconnected through plasma iron (transferrin)” [1].
Treatment with iron supplements
The daily requirement for iron is for children up to 3 months - 1.7 mg, from 4 to 6 months - 4.3 mg, from 7 to 12 months - 7.8 mg, up to 3 years - 6.9 mg, from 4 to 6 years old - 6.1 mg, from 7 to 10 years old - 8.7 mg, in girls from 11 years old - 14.8 mg, in boys from 11 to 18 years old - 11.3 mg, after 18 years old - 8.7 mg; in women under 50 years old - 14.8 mg (and more if there is blood loss), after 50 years old - 8.7 mg. During the day, about 0.1–0.3 mg is excreted in urine and 4–16 mg in feces; 10–50 mg of iron is lost with menstruation. Iron requirements in women are 30–90% higher than in men; during pregnancy, the need increases by approximately 60% [12].
When treating with iron preparations for oral use, the following general principles are usually taken into account. Normally, about 7–10% of iron administered orally is absorbed, with depletion of its reserves - up to 17%, and with iron deficiency anemia - up to 25%. The maximum amount of iron incorporated into erythroblasts and used for hemoglobin synthesis is about 25–30 mg per day. Increasing the daily dose above 200 mg (in terms of elemental iron) significantly increases the frequency and severity of adverse reactions. When treating anemia, it is usually recommended to take 50–60 mg of elemental iron orally. This dose is divided into two or three doses; Duration of therapy is up to 3 months. The following preparations are often used: ferrous fumarate, 325 mg tablet (elemental iron content 108 mg); ferrous sulfate, tablet 325 mg (elemental iron content 65 mg); Ferrous gluconate, tablet 325 mg (elemental iron content 35 mg).
Typically, blood hemoglobin levels begin to increase by 0.7–1.0 g/dL per week approximately 2–3 weeks after the start of therapy. Treatment with iron supplements is considered adequate if serum ferritin levels reach 50 μg/L. Due to the uncontrolled absorption of iron salts, which depends on the pH of the stomach contents, it is not always possible to predict how much iron enters the blood - insufficient, sufficient or excessive. If, in case of iron deficiency anemia, treatment with iron for two months does not have an effect, it is necessary to clarify the diagnosis and exclude cancer of the digestive tract.
Simple carbohydrates improve iron absorption - lactose, fructose, sorbitol. Histidine, lysine, cysteine increase bioabsorption by forming chelate complexes with iron ions. Iron absorption is reduced with transfusion polycythemia, copper and nickel deficiency. Salts of phytic acid, phytates, contained in wholemeal unleavened dough, cereals and dark green vegetables, also bind iron ions in the stomach and prevent its absorption. Fiber and phosphates, phosphoproteins contained in eggs, hinder the absorption of iron. Milk may reduce the ability to absorb iron because milk lactoferrin binds free iron. Iron absorption is reduced when bound to the food preservative ethylenediaminetetraacetic acid (EDTA), which is added to many products: carbonated drinks, seasonings, mayonnaise, sauces.
Iron absorption is impaired when taking alkaline drugs, H2 blockers, and proton pump blockers. In addition, increasing the single dose of iron supplementation may also slightly reduce iron absorption.
Vitamin and mineral complexes for iron deficiency
Of interest is the drug Feroglobin-B12 syrup [13], produced (Great Britain). 5 ml (1 teaspoon of syrup) of the drug contains: vitamin B1 4 mg (thiamine 5 mg), vitamin B2 1 mg (riboflavin 1.32 mg), vitamin B6 (pyridoxine hydrochloride) 2 mg, vitamin B12 5 mcg, niacin (nicotinamide, vitamin B3) 8 mg, folic acid 150 mcg, vitamin C (ascorbic acid) 10 mg, pantothenic acid 2 mg, calcium glycerophosphate 10 mg, iron 7 mg (iron ammonium citrate 35 mg), zinc (sulfate) 5 mg , manganese 0.25 g (manganese sulfate 21.99 mg), copper 0.25 mg (copper sulfate 0.91 mg), iodine 40 mcg (potassium iodide 52.32 mcg), lysine hydrochloride 40 mg, honey 100 mg, malt extract 500 mg. The drug contains the optimal amount of elemental iron for complex vitamin preparations and a balanced composition of vitamins and microelements, which allows the use of Feroglobin-B12 for a long time without the risk of side effects.
Vitamin B1 (thiamine). The phosphorylated form of thiamine, thiamine pyrophosphate, is formed in the human body and is a precursor to enzymes that play a significant role in carbohydrate metabolism and, in particular, in the processes of decarboxylation of pyruvic acid. The recommended daily dose (RDD) is 1.1 mg. During pregnancy and breastfeeding, the daily dose increases to 1.5 mg.
Vitamin B2 (riboflavin). The biologically active form of riboflavin is flavin adenine dinucleotide, synthesized in the human body in the kidneys, liver and other tissues. Another riboflavin derivative, riboflavin-5-phosphoric acid, occurs naturally in yeast. Riboflavin ensures the normal course of redox processes in the body.
Vitamin B6 (pyridoxine) plays an important role in protein, carbohydrate and lipid metabolism, and is also involved in heme synthesis. The active forms of vitamin B6 are represented by a group of compounds derived from pyridine (pyridoxine (pyridoxole), pyridoxal and pyridoxamine), collectively called “pyridoxine”. The recommended daily dose is 1.5 mg. During pregnancy and breastfeeding, the RDA increases to 2.2 mg.
Vitamin C (ascorbic acid) is a water-soluble vitamin that has numerous functions. Chronic deficiency of vitamin C leads to disruption of collagen synthesis and can cause diseases such as scurvy. RSD – 60 mg. During pregnancy, the RDA increases to 70 mg, during breastfeeding - up to 95 mg.
Niacin (vitamin PP) is a water-soluble vitamin that takes part in redox reactions and interstitial respiration processes. It is found in poultry, fish and nuts. Deficiency leads to pellagra. RSD – 15 mcg. During pregnancy, the RDA increases to 17 mcg, and during breastfeeding – to 20 mcg.
Pantothenic acid gets its name from the Greek panthos, meaning “everywhere,” due to its extremely wide distribution in nature. Once in the body, it is converted into pantethine, which is part of coenzyme A, which plays an important role in the processes of oxidation and acetylation [14].
It has been convincingly shown that it is more advisable to use vitamin-mineral complexes, especially those containing microelements, in combination with iron in an amount comparable to the recommended daily dose, mainly to correct the content of nutrients in the body. In contrast, when treating patients with anemia as a result of blood loss, iron salts are needed in increased dosages. Such therapy will be successful only if the functional activity of the bone marrow is preserved.
Conclusion
Iron is a very common element in nature, including in food products. Iron is an essential component involved in the processes of maturation and differentiation of red blood cells from a stem cell to a normocyte. In healthy individuals, the amount of iron resorbed in the intestines does not depend on the body's needs. As a result, prolonged high iron absorption leads to siderosis. Excess iron is deposited in cells and tissues in the form of ferritin, a molecule that binds about 45,000 iron atoms. It is necessary to distinguish between the physiological consumption of iron in the body and the pathological loss of iron during bleeding.
The main task in the treatment of patients with anemia is to exclude the cause of the disease, which may be associated with chronic blood loss, a tumor (stomach or intestinal cancer), blood disease, etc. In case of iron deficiency, iron reserves are restored by nutritional means or after blood transfusions or red blood cell transfusions for iron account of the destroyed injected red blood cells. For moderate blood loss, blood transfusions are not necessary. Restoration of circulating blood after blood loss depends on the partial pressure of oxygen in the circulating blood, the functional state of the bone marrow, iron reserves in the body and a number of other reasons.
It is obvious that the so-called physiological iron deficiency anemia, for example, in women, does not arise as a result of iron loss during bleeding, but as a result of a disorder in the regulation of erythropoiesis of humoral genesis. Iron deficiency in this case is one of the syndromes that may not manifest itself clinically.
Regardless of the detection method and severity, hyposiderosis is not an independent “disease”, but a symptom of some disease: from moderate indigestion to circulatory pathology, hematopoiesis, malignant tumor, intoxication, etc.
Multi-month therapy with iron supplements for torpid posthemorrhagic anemia is irrational.
In such cases, it is necessary to find out the cause of the suppression of bone marrow functions and try to influence it. The dose of iron in a drug prescribed for both preventive and therapeutic purposes must correspond to the daily requirement for iron. Further increase in dose is ineffective, and excessive accumulation of iron in the body can lead to undesirable reactions. In most cases, with iron deficiency, the use of vitamin and mineral complexes containing iron is sufficient.
Test for iron in the blood (Fe, Iron)
The level of iron in the blood is determined as part of a biochemical blood test. The unit used for this is micromoles per liter (µmol/l). For this purpose, venous or capillary blood is analyzed. The research method is photometric.
The simplest and most accessible way to assess iron content in the body is a clinical blood test, which is taken from a finger. In this case, you can estimate the hemoglobin level in grams per liter.
Refining the analysis
A hemoglobin blood test is an important but not sufficient procedure for assessing iron levels in the blood.
It happens that hemoglobin is normal, but iron levels are low. In this case, it is necessary to analyze additional parameters that will indicate the true content of the trace element in the body. It is noteworthy that the amount of serum iron is an indicator that varies depending on the day and even time of day. To minimize inaccuracies, determining the level of iron in the blood is usually combined with other tests. Among these are an analysis of total serum iron-binding capacity (TIBC), as well as tests for ferritin and transferrin. The indicators of THC and transferrin are used to determine the amount of iron that is currently transported in the blood.
Indications
The level of iron in the blood is determined for the following purposes:
- for diagnosis, differential diagnosis and control of treatment of various types of anemia;
- for diagnosing hemochromatosis, a hereditary disease in which iron metabolism is disrupted (there is too much of it);
- to determine transferrin saturation with iron (find out exactly how much iron the blood carries);
- to monitor the effectiveness of treatment with iron preparations.
The study is carried out if the following diseases and conditions are suspected:
- if there are deviations in the results of the general analysis (hemoglobin level, red blood cells, hematocrit);
- in case of poisoning with salts or drugs (overdose) of iron;
- if iron deficiency is suspected;
- for diseases of the gastrointestinal tract leading to impaired absorption of iron in the small intestine;
- with malnutrition (when the diet lacks iron, and this leads to adverse consequences);
- if you suspect hereditary pathologies accompanied by impaired iron metabolism;
- for various types of bleeding that can lead to the loss of a significant amount of iron (for example, heavy bleeding during menstruation, stomach ulcers and other conditions and diseases).
criteria for an excess of a substance in the body?
In such a situation, we recommend that you contact specialists who treat metabolic dysfunctions. A medical professional will help you find the causes, develop a personal research and treatment plan, and establish strict monitoring of your health.
Methods for treating excess iron :
- compliance with dietary nutrition,
- refusal of alcoholic drinks,
- use of antioxidants.
You should always remember that iron is not removed from the body, but remains in it for life!
This is why it is so important to detect this in the early stages and prevent the spread of dangerous substances to stop the functioning of the liver and many other organs, forming liver cancer, which will help you extend the years of your life.
Preparing for analysis
Laboratory blood tests often require some preparation to obtain correct results. An analysis of iron levels in the blood (Fe, Iron) is no exception. As a rule, these are the following requirements (which doctors and diagnostic laboratory staff will definitely inform the person about):
- Blood must be donated on an empty stomach.
- 12 hours before the expected test, you should avoid eating fatty foods, smoking and drinking alcohol. Strenuous physical activity is also not recommended.
- If you have to conduct instrumental studies, then you need to donate blood before performing an X-ray or computed tomography.
- A week before donating blood, you should stop taking iron-containing medications. It is also recommended to discontinue other medications (if possible). If the medication cannot be delayed, the patient should tell the doctor, who will take this into account when interpreting the test results.
- If a person has had a blood transfusion, an iron test can only be done a few days after the blood transfusion.
There may also be other recommendations and restrictions for each patient. The doctor will definitely indicate them. It is important to strictly follow the prescribed rules, since the results of the analysis (and therefore the treatment regimen) depend on this.
During pregnancy, women often experience iron deficiency in the body. Photo: freeograph / freepik.com
Normal blood iron levels for men, women and children
The normal level of iron in the blood is the amount at which the body functions fully. This indicator depends on a number of factors, including age and gender. Therefore, normal iron levels differ between children, women and men.
Iron levels during pregnancy
Separately, it is necessary to say about the norms of iron in the blood during pregnancy.
This period in a woman’s life is accompanied by serious changes not only in hormonal levels, but also in biochemical blood parameters, including iron. During pregnancy, the need for iron (as well as other macro- and microelements) increases. This is mainly due to the fetal needs for these substances. In addition, a pregnant woman's blood volume increases by about 30%. This means that the need for hemoglobin components (iron and amino acids) also increases.
It is worth considering that as the fetus grows, the physiological daily consumption of iron will increase. So, if in the first trimester the additional need for iron is estimated at 0.8 mg per day, then in the second trimester it is 5 mg, and in the third – up to 6-7 mg. During pregnancy, on average, a woman needs an additional 800-900 mg of iron.
Normal serum iron levels for a pregnant woman are 13-30 µmol per liter.
From the point of view of the body's supply of iron, the most vulnerable categories are considered to be pregnant women, as well as children and adolescents during the period of active growth and development.
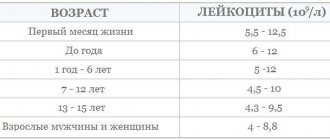
Iron levels are also affected by physical activity, so people involved in sports also need to monitor this indicator. Table 1 shows the reference (normal) values of serum iron in the blood in micromoles per liter in women and men of different age groups. Table 1. Norm of iron content (µmol/l) in children of different ages, women and men
| Age | Floor | Iron norm µmol/l |
| less than 1 month | female | 5,1 – 22,6 |
| male | 5,6 – 19,9 | |
| from 1 to 12 months | female | 4,6 – 22,5 |
| male | 4,9 – 19,6 | |
| from 1 to 4 years | female | 4,6 – 18,2 |
| male | 5,1 – 16,2 | |
| from 4 to 7 years | female | 5,0 – 16,8 |
| male | 4,6 – 20,5 | |
| from 7 to 10 years | female | 5,5 – 18,7 |
| male | 4,9 – 17,3 | |
| from 10 to 13 years | female | 5,8 – 18,7 |
| male | 5,0 – 20,0 | |
| from 13 to 16 years old | female | 5,5 – 19,5 |
| male | 4,8 – 19,8 | |
| from 16 to 18 years old | female | 5,8 – 18,3 |
| male | 4,9 – 24,8 | |
| over 18 years old | female | 6,6 – 26 |
| male | 11,6 – 28 |
Causes of high iron
An increased level of iron is said to be when its concentration is more than 28-30 µmol/l (reference values may differ slightly in different laboratories).
The following diseases and conditions can be the causes of elevated iron levels in the blood:
- Hemochromatosis. This is a hereditary disease in which the rate of iron accumulation exceeds the rate of its removal from the body2. Due to such disorders, iron accumulates in tissues, which leads to a number of other diseases. For example, against the background of hemochromatosis, joints may become inflamed (arthritis), sugar levels may rise, and even cirrhosis of the liver may develop.
- Some types of anemia. If with iron deficiency anemia there is a lack of iron, then with hemolytic or sideroblastic anemia there is an excess. In hemolytic anemia, red blood cells are intensively destroyed. This results in the release of heme iron into the blood. With sideroblastic anemia, iron metabolism in the bone marrow is disrupted, which causes hemoglobin synthesis to slow down.
- Iron poisoning. Most often, such poisoning occurs due to an overdose of iron supplements. To prevent this problem, you should take such medications only as prescribed by your doctor. Keep them away from children. For them, the toxic dose is much lower than for adults.
- Thalassemia. This is a hereditary disease in which the process of hemoglobin synthesis is disrupted. With thalassemia, less iron is used for the formation of hemoglobin, which leads to its excess in the blood.
- Blood transfusion. Several days after a blood transfusion, a person usually has an increased iron content.
- Premenstrual period. In women, serum iron levels increase slightly before menstruation. This is a normal physiological phenomenon that can mislead the doctor if you donate blood for analysis during this period. For this reason, women are recommended to donate blood after menstruation.
- Some diseases of internal organs. The level of iron in the blood is greatly influenced by the condition of the liver, spleen and pancreas. With diseases of these organs, there may also be a disturbance in iron metabolism, leading to its excessive accumulation in tissues.
- Excessive iron intake. This is most often observed when taking iron supplements. An excess of iron in the blood from food is unlikely.
What symptoms indicate high iron levels?
Elevated levels of iron in the blood can be manifested by the following symptoms:
- nausea and vomiting;
- bowel disorder (diarrhea or constipation);
- renal dysfunction;
- unexplained weight loss;
- loss of appetite;
- pain and swelling in the joints;
- joint inflammation (arthritis);
- elevated blood sugar levels;
- atherosclerosis;
- hair loss;
- muscle pain;
- decreased immunity (resulting in frequent infectious diseases);
- decreased sex drive;
- delayed physical and mental development in children.
If iron levels are elevated, blood donation is recommended. Photo: kasto / Depositphotos
How to suspect iron deficiency in a baby
The following symptoms may serve as alarm bells that allow parents to suspect iron deficiency:
- lethargy and weakness;
- increased irritability;
- tearfulness;
- decreased appetite;
- pale skin;
- frequent colds.
In such a situation, it is necessary to take a blood test and contact a pediatrician.
Lack of iron from the first months of a baby’s life affects his mental and physical development, causing serious delays and impairments.
Causes of low iron levels
Low iron levels may result from the following diseases and conditions:
- Insufficient intake of microelements from food. This is the most common cause of serum and heme iron deficiency. Passion for diets, as well as refusal to consume animal proteins (vegetarianism, veganism) only aggravate this problem.
- Increased iron consumption. This is most often observed in children during periods of active growth and development, as well as during pregnancy and breastfeeding.
- Infectious diseases. Acute or chronic infections often lead to a decrease in serum iron. This mechanism is evolutionary, since with a low iron content in tissues, cell division (including bacteria) slows down.
- Vitamin deficiency. B vitamins, vitamin C and folic acid are necessary for normal absorption of iron in the intestines. A deficiency of these vitamins leads to poor absorption of iron.
- Anorexia and starvation. A pathological desire to lose weight can lead to anorexia. A person refuses to eat, which quickly leads to exhaustion of the body.
- Dysgesia and parosmia. Distortion of smell (parosmia) and taste (dysgesia) is one of the possible reasons for refusing foods containing iron. For example, a person may become disgusted with meat and meat products. This problem is especially relevant during the coronavirus pandemic. One of the complications of infection is impaired sense of smell and taste.
- Diseases of the digestive tract. Such pathologies impair the absorption of iron in the small intestine.
- Worms. Parasites feed on the host's nutrients and minerals. And some types of worms (for example, roundworms) also drink blood, which also leads to loss of hemoglobin and serum iron.
- Pregnancy and breastfeeding. Particular attention should be paid to the third trimester of pregnancy, when the additional consumption of iron is maximum. It should also be taken into account that the child receives iron through mother’s milk. Therefore, during lactation you also need to take care of getting an adequate amount of iron from food and/or medications.
- Substances that slow down the absorption of iron. These include calcium, vitamin E, zinc and oxalates. Iron absorption is also impaired by coffee and tea.
- Intoxication. Donating blood for iron is also not recommended immediately after infectious and inflammatory diseases. This is due to the fact that during intoxication, iron consumption increases. To neutralize toxins, the liver intensively produces enzymes, an integral part of which is iron.
- Bleeding. This may include bleeding from open wounds, internal bleeding, or heavy menstruation.
Low hemoglobin with normal serum iron levels
We wrote above that normal hemoglobin does not at all indicate a normal level of iron in the blood.
However, the opposite situation is also possible when, with normal or elevated iron levels, the patient has low hemoglobin. For the synthesis of hemoglobin, you need not only iron, but also folic acid and vitamin B123. A lack of these vitamins (even with normal or elevated levels of iron in the blood) leads to a decrease in hemoglobin levels. This occurs when the absorption of vitamins is impaired, their consumption is increased (for example, during pregnancy, childbirth, dysbacteriosis, helminthic infestations, taking certain medications) or insufficient intake from food.
What symptoms indicate low iron levels in the blood?
There are latent and pronounced signs of iron deficiency in the body. The latent stage of iron deficiency is observed with a slight decrease in the concentration of the microelement. This may be accompanied by the following symptoms:
- increased fatigue;
- malaise;
- weakness;
- decreased concentration;
- cardiopalmus;
- brittle nails;
- emotional disorders (depressive states);
- headache and dizziness;
- irritability; pale skin;
- difficulty swallowing;
- hair loss;
- sleep disorder;
- deterioration of memory and cognitive functions.
If iron deficiency is not corrected, symptoms worsen. At the stage of pronounced manifestations it is:
- Decreased immunity. The patient often suffers from acute respiratory diseases.
- Cold extremities. Blood microcirculation deteriorates, causing a person’s feet and hands to freeze. Body temperature may also drop below 36.6 degrees.
- Digestive disorders. There are problems with stool (constipation or diarrhea), flatulence, heartburn, abdominal pain and other symptoms of disorders of the gastrointestinal tract.
- Neurological disorders. A person's mood changes. Irritability and short temper appear. Headaches are common.
- Dyspnea. With a lack of iron, oxygen transport deteriorates. Oxygen starvation occurs, which causes shortness of breath and tachycardia.
How to lower or increase iron levels in the blood
Excess and lack of iron is a problem that is fraught with the development of many diseases. In both cases, it is possible to achieve normalization of indicators.
If the level of iron in the blood is low, you need to choose foods that contain a lot of this trace element. Photo: antoninavlasova / freepik.com
If your iron level is elevated, you can normalize it using the following measures:
- Drug treatment. The patient is prescribed medications that accelerate the removal of iron from the body. For example, these are active substances that bind to iron - deferoxamine and calcium thetacine. In addition, iron excretion is accelerated when taking hepatoprotectors and zinc preparations.
- Diet. If you have high iron levels, it is recommended to limit your consumption of foods rich in this trace element. These are meat, mushrooms, beans, cocoa and seafood. It is noteworthy that B vitamins, vitamin C and folic acid improve the absorption of iron into the blood. Therefore, it is recommended to avoid taking these vitamins. But tea, coffee and calcium impair iron absorption. If you have no contraindications, you can introduce these calcium-rich drinks and foods into your diet.
- Blood donation. For some patients, regular bloodletting is recommended if iron levels are high. The best choice for the patient would be donation, because the selected blood will save someone’s life.
- Treatment with leeches. Iron concentrations can also be reduced through hirudotherapy (leech treatment).
- Blood transfusion. As a rule, exchange transfusion is used for iron poisoning. The patient is phlebotomized while receiving donor blood.
Most often, people experience iron deficiency, not excess. Therefore, methods of raising iron levels in the blood are more relevant.
First of all, it is necessary to find out the reason for the decrease in iron in the blood. If you take iron supplements without curing the underlying disease, the normalization of iron in the blood will be short-term.
As a rule, if we are talking about a slight lack of iron, then the indicators can be normalized with the help of proper nutrition. Your doctor will advise you to eat more meat. For example, 100 g of beef contains 2.7 mg of iron. And the most of this microelement is in pork liver - up to 19 mg.
Note that heme iron (its sources are meat products) is absorbed much better than non-heme iron. The latter is relatively abundant in mushrooms, dried fruits, beans, peas, cocoa, buckwheat and nuts.
If there is a significant lack of iron in the blood, patients are prescribed appropriate medications. They are used in the form of tablets, solutions, intramuscular or intravenous injections.
Iron intake standards for patients with anemia
Sometimes tests reveal not just a lack of iron, but a more serious situation - iron deficiency anemia. Typically, this condition develops against the background of gastrointestinal diseases, tumor processes, helminthic infestations, and constant bleeding. It is often diagnosed in people who have experienced significant blood loss during injury or surgery.
Iron deficiency anemia is diagnosed if the hemoglobin level drops to 100–70 g/l and serum ferritin drops to 15 ng/ml.
If iron deficiency anemia is diagnosed, you cannot prescribe treatment yourself. The doctor selects the therapy. Perhaps he will prescribe multivitamin complexes and dietary supplements with iron, and in the most severe cases, he may even prescribe iron supplements - quite strong drugs with numerous side effects. You can take such medications only under the supervision of your doctor.