Allergodil (vial-drops eye drops 0.05% 6ml)
A country
Italy
The country of production may vary depending on the batch of goods. Please check with the operator for detailed information when confirming your order.
Active substance
Azelastine
Compound
1 bottle contains: Volume of solution in the bottle 6 ml\10 ml Active substance: Azelastine hydrochloride 3,000 mg\5,000 mg Excipients: hypromellose 6,000 mg\10,000 mg, disodium edetate 3,000 mg\5,000 mg, benzalkonium chloride 0.750 mg\1.25 0 mg, sorbitol 399.996 mg\666.660 mg, sodium hydroxide up to pH 6.0\up to pH 6.0, water for injection up to 6 ml (6,090 g)\Up to 10 ml (10,150 g)
Pharmacological properties
Azelastine, a phthalazinone derivative, is a strong long-acting antiallergic agent that selectively blocks H1 receptors.
When applied to the ocular mucosa, additional anti-inflammatory and membrane-stabilizing effects of azelastine appear: azelastine inhibits the release of mediators of the early and late phases of allergic reactions, for example, leukotriene, histamine, platelet activating factor; reduces the number of ICAM-1 (intercellular adhesion molecule 1) and eosinophil cells. No clinically significant effect on the QT (QTc) interval was detected even with long-term use of high doses of azelastine. Pharmacokinetics Even with repeated use of Allergodil eye drops (1 drop in each eye four times a day), the maximum concentrations of azelastine in the blood plasma are very low and are detected at or below the limit of measurement.
Indications for use
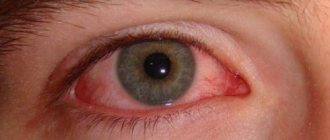
— prevention and treatment of seasonal allergic conjunctivitis; - treatment of non-seasonal (year-round) allergic conjunctivitis.
Contraindications
- Hypersensitivity to the active substance or other components of the drug; — Age up to 4 years; — First trimester of pregnancy.
Use during pregnancy and lactation
When tested on animals, an undesirable effect on the fetus was found when the drug was administered orally in doses many times higher than the therapeutic dose, and therefore the use of the drug in the first trimester of pregnancy is not recommended, and in the second and third trimesters and during lactation is possible only in case of emergency, if the possible benefit to the mother outweighs the risk to the fetus/child.
Directions for use and doses
Seasonal allergic conjunctivitis: unless otherwise recommended by a doctor, adults and children over 4 years old should instill 1 drop into each eye 2 times a day (morning and evening). If necessary, the dose is increased to 4 times a day, one drop in each eye. The drug is used until symptoms disappear, but not more than 6 weeks. In case of suspected exposure to an allergen, the drug is used for prophylactic purposes. Non-seasonal (year-round) allergic conjunctivitis: unless otherwise recommended by a doctor, adults and children over 4 years old should instill 1 drop into each eye 2 times a day (morning and evening). If necessary, the dose is increased to 4 times a day, one drop in each eye. Duration of use – no more than 6 weeks.
Side effects
The incidence of side effects is determined as follows: Very common: > 1/10;
Often: 1/100; Uncommon: 1/1000; Rarely: 1/10000; Very rare: Local: often - transient blurred vision, conjunctivitis; rarely – a burning sensation in the eye. General: infrequently - a feeling of bitterness in the mouth; very rarely - allergic reactions, headache, asthma, dyspnea, fatigue, flu-like symptoms, pharyngitis, itching, rhinitis.
Overdose
There are no data on drug overdose.
Interaction with other drugs
Not found. In case of concomitant therapy with other eye drops, they should be instilled into the eyes at intervals of at least 15 minutes.
special instructions
It is not recommended to wear contact lenses when using eye drops.
Impact on the ability to drive vehicles
In case of blurred vision immediately after instillation of the drug, you should refrain from driving a car or engaging in activities that require increased attention until it recovers.
Release form
Eye drops 0.05%. 6 ml or 10 ml of solution in a translucent high-density polyethylene bottle with a low-density polyethylene dropper and a white high-density polyethylene cap. 1 bottle along with instructions for use is placed in a cardboard box.
Storage conditions
At a temperature not higher than 25 ºС. Keep out of the reach of children.
Best before date
3 years. After opening the bottle, the drug should be used within 4 weeks. Do not use after the expiration date indicated on the package.
Conditions for dispensing from pharmacies
Over the counter.
Allergodil eye drops 0.05% 6ml No.1
Name
Allergodil.
Release form
Eye drops.
Dosage
0.05% 6 ml. Packing quantity: 1 pc.
Manufacturer
Meda Pharma.
INN
Azelastine.
FTG
H1-histamine receptor blocker.
Description
A transparent, colorless solution that does not contain visible mechanical inclusions.
Compound
1 bottle (6 ml) contains: Active ingredient: azelastine hydrochloride 3 mg. Excipients: hypromellose, disodium edetate, benzalkonium chloride, sorbitol, sodium hydroxide, water for injection.
Pharmacotherapeutic group
Drugs used in ophthalmology. Drugs used to eliminate inflammatory edema (decongestants) and other antiallergic drugs. Other antiallergic drugs. ATX code: S01GX07
Pharmacological properties
Azelastine, a phthalazinone derivative, is a strong long-acting antiallergic agent that selectively blocks Hi-receptors. When applied to the ocular mucosa, additional anti-inflammatory and membrane-stabilizing effects of azelastine appear. In vivo (preclinical data) and in vitro data indicate that azelastine inhibits the synthesis or release of early and late phase chemical mediators of allergic reactions, such as leukotriene, histamine, platelet activating factor and serotonin. To date, ECG evaluation during long-term therapy in patients taking high doses of oral azelastine has shown that there is no clinically significant effect of azelastine on the QT interval in multiple-dose studies. There was no association of azelastine with the occurrence of ventricular arrhythmia or torsade de pointes in 3,700 patients treated with oral azelastine. Relief of symptoms of allergic conjunctivitis should be noted within 15-30 minutes.
Indications for use
prevention and treatment of symptoms of seasonal allergic conjunctivitis in adults and children 4 years of age and older; treatment of symptoms of non-seasonal (year-round) allergic conjunctivitis in adults and children 12 years of age and older.
Contraindications
Hypersensitivity to the active substance or other components of the drug.
Directions for use and doses
Seasonal allergic conjunctivitis: unless otherwise recommended by a doctor, adults and children over 4 years old should instill a drop into each eye 2 times a day (morning and evening). If necessary, the dose is increased to 4 times a day, one drop in each eye. In case of suspected exposure to an allergen, the drug is used for prophylactic purposes. Non-seasonal (year-round) allergic conjunctivitis: unless otherwise recommended by a doctor, adults and children over 12 years old should instill 1 drop into each eye 2 times a day (morning and evening). If necessary, the dose is increased to 4 times a day, one drop in each eye. Based on safety and effectiveness demonstrated in clinical studies over a period of up to 6 weeks, the duration of any course of treatment should not exceed 6 weeks. Relief of symptoms of allergic conjunctivitis should be observed within 15-30 minutes. If symptoms worsen or last longer than 48 hours without improvement, you should consult a doctor. If one or more doses of eye drops are missed, they should be used as soon as the patient remembers. The next dose should be taken at the usual time. Patients should not take a double dose to make up for a missed dose. Interrupting or stopping treatment with Allergodil eye drops will likely cause symptoms to return.
Side effect
Information on adverse reactions is presented in accordance with systemic organ classification and frequency of occurrence. Frequency categories were determined according to the following classification: very common (>1/10), common (>1/100, but 1/1,000, but 1/10,000, but
Overdose
Characteristic reactions after an overdose are unknown; overdose reactions are unlikely. There is no experience with toxic doses of azelastine hydrochloride in humans. Based on animal studies, central nervous system effects should be expected in cases of overdose or intoxication. Treatment of such disorders should be symptomatic. There is no known antidote. Interaction with other medicinal products and other types of interactions No relevant studies have been conducted with Allergodil eye drops. Studies have been conducted to examine the interactions of high oral doses of azelastine, but these are not applicable to Allergodil eye drops as systemic levels after administration of eye drops are in the picogram range.
Precautionary measures
This drug is not intended to treat eye infections. Allergodil eye drops contain 0.00375 mg of benzalkonium chloride in each drop, which is equivalent to 0.00375 mg/0.03 ml. Benzalkonium chloride can be absorbed by soft contact lenses and may change the color of the contact lenses. Contact lenses should be removed before use and put back on at least 15 minutes after use. Benzalkonium chloride may also cause eye irritation, especially if you have dry eye syndrome or disease of the cornea (the clear layer at the front of the eye). If you experience an abnormal sensation in the eye, burning or pain after using this medicine, you should consult a doctor.
Impact on the ability to drive vehicles and operate machinery
After using Allergodil eye drops, mild short-term irritation may occur; a more significant effect on vision is unlikely. However, if any transient effects on vision are observed, the patient is advised to wait until these effects disappear before operating machinery.
Fertility, pregnancy and lactation
Fertility The effect on fertility in humans has not been studied. Pregnancy There is insufficient information regarding the safety of azelastine during pregnancy in women. High doses of oral azelastine have been shown to cause adverse effects (fetal death, growth retardation, and skeletal malformation) in animals used in experimental studies. Application as eye drops (topically) will result in minimal systemic exposure. However, caution should be exercised when using Allergodil eye drops during pregnancy. Lactation Azelastine passes into breast milk in small quantities. Therefore, it is not recommended to use Allergodil eye drops during lactation.
Package
Eye drops, solution 0.05%. 6 ml of solution in a translucent high-density polyethylene bottle with a low-density polyethylene dropper and a white high-density polyethylene cap. 1 bottle along with instructions for use is placed in a cardboard box.
Storage conditions
At a temperature no higher than . Keep out of the reach of children. Shelf life: 3 years. After opening the bottle, the drug should be used within 4 weeks. Do not use after the expiration date indicated on the package.
Vacation conditions
Over the counter.
Buy Allergodil eye drops (solution) 0.05% in 6 ml bottle in pack No. 1 in the pharmacy
Price for Allergodil eye drops (solution) 0.05% in bottle 6 ml in pack No. 1
Instructions for use for Allergodil eye drops (solution) 0.05% in 6 ml bottle in pack No. 1
Allergodil eye drops 0.05% 6ml
Allergodil eye drops 0.05% 6ml
Tradename:
ALLERGODIL®
International nonproprietary name:
Azelastine
Dosage form:
eye drops
Compound:
1 bottle contains:
| Compound | Volume of solution in the bottle | |
| 6 ml | 10 ml | |
| Active substance: Azelastine hydrochloride | 3,000 mg | 5,000 mg |
| Excipients: | ||
| Hypromellose | 6,000 mg | 10,000 mg |
| Disodium edetate | 3,000 mg | 5,000 mg |
| Benzalkonium chloride | 0.750 mg | 1,250 mg |
| Sorbitol | 399.996 mg | 666.660 mg |
| Sodium hydroxide | up to pH 6.0 | up to pH 6.0 |
| Water for injections | Up to 6 ml (6,090 g) | Up to 10 ml (10.150 g) |
Description:
Colorless, transparent or almost transparent solution.
Pharmacotherapeutic group:
Antiallergic agent - H1-histamine receptor blocker.
Pharmacological properties
Azelastine, a phthalazinone derivative, is a strong long-acting antiallergic agent that selectively blocks H1 receptors. When applied to the ocular mucosa, additional anti-inflammatory and membrane-stabilizing effects of azelastine appear: azelastine inhibits the release of mediators of the early and late phases of allergic reactions, for example, leukotriene, histamine, platelet activating factor; reduces the number of ICAM-1 (intercellular adhesion molecule 1) and eosinophil cells.
No clinically significant effect on the QT (QTc) interval was detected even with long-term use of high doses of azelastine.
Pharmacokinetics
Even with repeated use of Allergodil eye drops (1 drop in each eye four times a day), the maximum concentrations of azelastine in the blood plasma are very low and are detected at or below the limit of measurement.
Indications for use:
— prevention and treatment of seasonal allergic conjunctivitis; - treatment of non-seasonal (year-round) allergic conjunctivitis.
Contraindications
- Hypersensitivity to the active substance or other components of the drug; — Age up to 4 years; — First trimester of pregnancy.
Use during pregnancy and lactation
When tested on animals, an undesirable effect on the fetus was found when the drug was administered orally in doses many times higher than the therapeutic dose, and therefore the use of the drug in the first trimester of pregnancy is not recommended, and in the second and third trimesters and during lactation is possible only in case of emergency, if the possible benefit to the mother outweighs the risk to the fetus/child.
Directions for use and doses
Seasonal allergic conjunctivitis: unless otherwise recommended by a doctor, adults and children over 4 years old should instill 1 drop into each eye 2 times a day (morning and evening). If necessary, the dose is increased to 4 times a day, one drop in each eye. The drug is used until symptoms disappear, but not more than 6 weeks.
In case of suspected exposure to an allergen, the drug is used for prophylactic purposes.
Non-seasonal (year-round) allergic conjunctivitis: unless otherwise recommended by a doctor, adults and children over 4 years old should instill 1 drop into each eye 2 times a day (morning and evening). If necessary, the dose is increased to 4 times a day, one drop in each eye.
Duration of use – no more than 6 weeks.
Side effects
The incidence of side effects is determined as follows:
Very common: > 1/10; Often: 1/100; Uncommon: 1/1000; Rarely: 1/10000; Very rarely:
Local:
often - transient blurred vision, conjunctivitis; rarely – a burning sensation in the eye.
Are common:
infrequently - a feeling of bitterness in the mouth; very rarely - allergic reactions, headache, asthma, dyspnea, fatigue, flu-like symptoms, pharyngitis, itching, rhinitis.
Overdose
There are no data on drug overdose.
Interaction with other drugs
Not found. In case of concomitant therapy with other eye drops, they should be instilled into the eyes at intervals of at least 15 minutes.
special instructions
It is not recommended to wear contact lenses when using eye drops.
Impact on the ability to drive vehicles
In case of blurred vision immediately after instillation of the drug, you should refrain from driving a car or engaging in activities that require increased attention until it recovers.
Release form
Eye drops 0.05%. 6 ml or 10 ml of solution in a translucent high-density polyethylene bottle with a low-density polyethylene dropper and a white high-density polyethylene cap. 1 bottle along with instructions for use is placed in a cardboard box.
Storage conditions
At a temperature not higher than 25 ºС. Keep out of the reach of children.
Best before date
3 years. After opening the bottle, the drug should be used within 4 weeks. Do not use after the expiration date indicated on the package.
Conditions for dispensing from pharmacies:
over the counter.