Pharmacodynamics and pharmacokinetics
Pharmacodynamics
Vizomitin was created by academician Vladimir Petrovich Skulachev.
Eye drops contain plastoquinonyldecyltriphenylphosphonium bromide (PDTP) , a derivative of plastoquinone containing triphenylphosphine . In low concentrations, the substance has a strong antioxidant and stimulating effect on epithelization, tear production, and increasing the stability of the tear film.
Pharmacokinetics
No studies have been conducted on the pharmacokinetics of the drug in humans. In animal studies, it was found that the distribution of PDTP occurred within two days after intravenous administration. The drug was present mainly in the tissues of the liver, kidneys and heart in the first hour after administration. PDTP quickly undergoes metabolic degradation and reacts with plasma proteins.
Instructions for use
MINISTRY OF HEALTH OF THE RUSSIAN FEDERATION
INSTRUCTIONS for medical use of the drug
___Visomitin®__ name of the medicinal product
Registration number : LP-001355
Trade name : Visomitin®
International nonproprietary or generic name : Plastoquinonyldecyltriphenylphosphonium bromide
Dosage form : Eye drops
Composition per 1 ml : Active ingredient: Plastoquinonyldecyltriphenylphosphonium bromide (PDTP) 0.155 mcg Excipients: Benzalkonium chloride 0.1 mg, hypromellose 2 mg, sodium chloride 9 mg, sodium dihydrogen phosphate dihydrate 0.81 mg, sodium hydrogen phosphate dodecahydrate 1.16 mg, sodium hydroxide 1 M solution to pH 6.0 – 8.0, purified water to 1 ml.
Description Clear or slightly opalescent, colorless or slightly colored liquid.
Pharmacotherapeutic group Keratoprotective agent. Antioxidant agent.
ATX code : S01ХA
Pharmacological properties Pharmacodynamics Plastoquinonyldecyltriphenylphosphonium bromide (PDTP) is a derivative of plastoquinone, which is linked to a triphenylphosphine residue through a linker chain (C10). When used in low (nanomolar) concentrations, PDTP exhibits high antioxidant activity. It has a stimulating effect on the process of epithelization of the cornea, helps to increase the stability of the tear film.
One of the reasons for the development of age-related cataracts is the damaging effect of ultraviolet radiation, which initiates photo-oxidation processes leading to denaturation of the main structural components of the lens - crystallins. The first protection of eye tissue from ultraviolet radiation is tear fluid, which absorbs ultraviolet light in the range of 240-320 nm and neutralizes it due to the components of the antioxidant activity of tear fluid. According to preclinical studies, the anti-cataract effect of the drug Visomitin® is associated with an increase in the level of expression of the main lens proteins α-crystallins. According to a clinical study, patients with age-related cataracts who used Visomitin® showed an increase in the antioxidant activity of tears.
Pharmacokinetics Preclinical studies on animals have shown that when using Visomitin® eye drops in the form of instillations, PDTP is not detected in the blood. Following oral administration of PDTP to healthy volunteers, the plasma half-life is approximately 45 minutes.
Indications for use Including, as part of complex therapy: Symptoms of dry eye syndrome/corneal-conjunctival xerosis (poor tolerance to wind, conditioned air, smoke, foreign body sensation, pain, burning, dry eyes, floating visual acuity, lacrimation, redness of the eyes in the area not covered by the eyelids). Symptoms of the initial stage of age-related cataracts (gradual blurring and decreased visual acuity).
Contraindications Hypersensitivity to the components of the drug. Children under 18 years of age (no clinical trial data available).
Use during pregnancy and breastfeeding There is no data on the safety of using Visomitin® during pregnancy. Studies of generative function and teratogenic effect in rats did not reveal signs of impaired fertility or defects in fetal development caused by PDTF.
It is unknown whether PDTP is excreted in breast milk. Appropriate studies have not been conducted in lactating women. The use of Visomitin® during pregnancy is possible if the expected benefit to the mother outweighs the potential risk to the fetus. If you are pregnant, think you might be pregnant, or are planning a pregnancy, you should consult your doctor.
Method of administration and dosage Symptoms of “dry eye” syndrome: 1-2 drops of the drug into the conjunctival sac 3 times a day. According to clinical studies, the therapeutic effect is achieved in the first two to four weeks of use. The therapeutic effect is persistent when used for 6 weeks. If there is no effect during the first two weeks of treatment, examination by a specialist is necessary. The initial stage of age-related cataracts: 1-2 drops of the drug into the conjunctival sac 3 times a day. The duration of treatment is 6 months. The diagnosis of “initial stage of age-related cataracts” must be made by a specialist. If there is no improvement after treatment, or symptoms worsen, or new symptoms appear, you should consult your doctor. Use the drug only according to the indications, the method of use and in the doses indicated in the instructions for use.
Side effects Classification of adverse reactions by organs and systems, indicating the frequency of their occurrence: very often (≥10), often (≥1/100, <1/10), infrequently (≥1/1000, <1/100), rarely ( ≥1/10000, <1/1000), very rare (<1/10000), including isolated reports, frequency unknown (frequency cannot be estimated based on available data). On the part of the organ of vision Often: short-term pain and burning after instillation. Uncommon: temporary blurred vision. Other Often: allergic reactions (itching, redness and swelling of the eyelids and conjunctiva). If you experience the side effects listed in the instructions, or they get worse, or you notice any other side effects not listed in the instructions, tell your doctor.
Overdose When using the drug Visomitin® in accordance with the instructions for use, an overdose is unlikely. Excessive use may increase side effects. Treatment: symptomatic therapy.
Interaction with other medicinal products There have been no previously reported adverse interactions between Visomitin® and other medicinal products. If necessary, it can be used simultaneously with other eye drops; the interval between instillations should be at least 15 minutes.
Special instructions Contact lenses Visomitin® eye drops contain benzalkonium chloride as a preservative and are not recommended for use when wearing contact lenses. Before instilling eye drops, contact lenses should be removed and put back on no earlier than 15 minutes after using the drug. The bottle must be closed after each use. Do not touch the eye with the tip of the pipette while instilling. The active ingredient of Visomitin® is very sensitive to light: between uses, do not store the bottle in a lighted place! Patients using Visomitin® for the relief of symptoms of dry eye syndrome and cataract maintenance treatment without a prescription should consult a doctor in the following cases:
- use of the drug to relieve symptoms of dry eye syndrome for 6 weeks or more;
- the presence of purulent discharge from the eyes;
- redness of the areas of the eye covered by the eyelids;
- sudden (from one day to a month) deterioration of vision;
- the appearance of new symptoms or changes in previously observed symptoms affecting the organs of vision.
Patients suffering from recurring symptoms of dry eye or progressive vision loss due to cataracts for a long time should be regularly examined by an ophthalmologist.
Effect on the ability to drive vehicles and machinery If short-term blurred vision occurs after using the drug, it is not recommended to drive vehicles or engage in activities that require clear visual perception until it is restored.
Release form Eye drops with a concentration of 0.155 mcg/ml in polyethylene bottles of 5 ml or 10 ml with stoppers - droppers and screw caps. Each bottle with instructions for use is placed in a cardboard pack.
Storage conditions In a dark place at a temperature from +2 to +8 0C. Store the opened bottle in a place protected from light at a temperature from +2 to +8 0C; use within 1 month. Keep out of the reach of children!
Shelf life: 2 years. Do not use the drug after the expiration date indicated on the package.
Conditions of release Dispensed without a prescription.
Name, address of the manufacturer of the medicinal product and address of the place of production of the medicinal product 1. CJSC Framon, 121552, Moscow, st. 3rd Cherepkovskaya, 15a, building 7. 2. CJSC “Firn M”, 108804, Moscow, Kokoshkino settlement, settlement. Kokoshkino, st. Dzerzhinskogo, 4. Organization carrying out quality control: LLC "Research Institute of Mitoengineering of Moscow State University", 119992, Moscow, st. Leninskie Gory, vl. 1, page 73
Organization accepting complaints from consumers: Mitotech LLC, 119234, Moscow, st. Leninskie Gory, 1, building 77, floor 1, room 101A. Phone fax.
Analogs
Level 4 ATC code matches: Systain Ultra
Systain Gel
Systain Balance
Okoferon
Khilozar-Komod
Hilo-Chest
Visine Pure Tear
Restasis
Catalin
Quinax
Mirtilene Forte
Emoxy optic
Adgelon
Vita-Iodurol
Natural tear
Artificial tear
Optiv
Ophtolic
Balarpan
Taurine
Currently, there are no analogues of Vizomitin with an identical composition.
The article presents the results of an open randomized controlled comparative clinical study of the effectiveness and safety of the drug "Visomitin" in the treatment of patients with dry eye syndrome. Visomitin is the first drug with a mitochondria-targeted antioxidant as the main active ingredient. Visomitin has shown high effectiveness in the treatment of dry eye, which is confirmed by an improvement in subjective sensations, changes in visual acuity, normalization of the clinical picture, and improvement in diagnostic test parameters.
The first experience of using the drug Vizomitin in the treatment of “dry eyes”
Results on open randomized controlled comparative clinical trial on efficacy and safety of novel pharmaceutical Visomitin in the treatment of 'dry eye' patients are reported. Visomitin is the first pharmaceutical containing mitochondria-targeted antioxidant as an active substance. In this study Visomitin showed high efficacy in dry eye treatment. Results on recovery of subjective condition, visual acuity, clinical parameters and diagnostic tests data are reported.
The problem of dry eye syndrome (DES) has recently been widely covered in the ophthalmological literature. This disease combines a large number of pathological changes in the ocular surface and occurs in numerous patients not only with ophthalmological problems [1]. According to research conducted by the Johns Hopkins University International Task Force, DES occurs in 11% of people aged 30-60 years and in 15% over 65 years [2]. According to Russian authors, among patients over 50 years of age, dry eye syndrome occurs in more than 67% of cases [3]. Concomitant somatic pathology in old age is itself the cause of the manifestations of dry eye syndrome; the need to take various medications (such as antihypertensives, adrenergic agonists, antiarrhythmic drugs, antiparkinsonian drugs, etc.) also leads to a decrease in tear production [4, 5]. The most important aspect of the development of dry eye disease is concomitant ocular pathology, such as glaucoma and cataracts. The use of antihypertensive eye drops worsens the condition of the anterior part of the eye and aggravates the course of dry eye syndrome [6]. Modern capabilities of cataract surgery make it possible to perform operations in the early stages of the development of lens opacities, but in the postoperative period there are often problems with impaired tear production and, as a consequence, a decrease in the quality of life [7]. A certain proportion of patients with dry eye syndrome are pre- and menopausal women, which is associated with hormonal changes in the body [8].
Considering the above, it is obvious that with age there is an increase in the number of patients with dry eye syndrome. In addition, oxidative stress—damage to molecules, cells, and tissues by free radicals—plays a key role in the pathogenesis of dry eye syndrome. The tissues of the eye are most vulnerable to attack by free radicals and reactive oxygen species (ROS). One of the most powerful producers of endogenous ROS in our cells under stress conditions are mitochondria. Such ROS damage mitochondria, disrupting the functioning of the electron transport chain, which leads to an even greater increase in ROS production due to the reduction of molecular oxygen in the initial and middle links of this chain [9]. Thus, mitochondria are involved in a kind of “vicious circle” of oxidative stress in the cell.
At the same time, antioxidant drugs have not yet taken their rightful place in the treatment of dry eye syndrome and the main attention is still paid to tear replacement therapy. A method to combat oxidative damage was proposed back in the 60s, when the use of antioxidants began [10]. However, due to the presence of membranes, antioxidants are virtually unable to penetrate mitochondria and neutralize endogenous ROS produced in the electron transport chain. It was proposed to combine the antioxidant with the lipophilic cation alkyltriphenylphosphonium, which, due to its positive charge and ability to pass through biological membranes, was shown [11] to specifically accumulate in the mitochondria of living cells. The resulting substance, named SkQ1 (INN - plastoquinonyldecyltriphenylphosphonium bromide), specifically accumulates in mitochondria, exhibits high antioxidant activity and is a regenerated antioxidant, because its spent (oxidized) form is reduced by the electron transport chain in vivo [12]. Preclinical studies have shown the effectiveness of this substance in models of a number of diseases [13, 14]. Plastoquinonyldecyltriphenylphosphonium bromide is the active substance of Visomitin eye drops, the world's first registered drug with a mitochondria-targeted antioxidant as the main active ingredient.
The purpose of the work was to study the effectiveness of using a new keratoprotective drug for the treatment of dry eye syndrome - Visomitin (registration number LP-001355), the world's first registered drug with a mitochondria-targeted antioxidant as the main active ingredient.
Materials and methods: a comparative study was conducted in which 40 patients took part, including 35 women and 5 men; 20 patients were treated with the drug “Visomitin” and 20 with the drug “Natural Tear” (the commercial name of a tear replacement drug produced for the treatment of dry eye syndrome by Alcon and which is one of the most popular drugs prescribed for this disease). The average age of the Visomitin group was 54.8±0.12 years, in the Natural Tears group - 53.8±0.15 years. All patients contacted the Institute with complaints and clinical presentations characteristic of DES. The first group was prescribed Visomitin in the form of instillations into the conjunctival cavity of the eye, 1 drop 3 times a day. Patients of the second group received eye drops “Natural Tear”, 1 drop 3 times a day. The course of treatment is 21 days with control visits on the 2nd, 3rd, 7th, 14th and 21st days of treatment.
Evaluation of the effectiveness of treatment was carried out according to the following criteria: dynamics of the functional state of the organ of vision and visual acuity indicators; dynamics of the Norn test; improvement of Schirmer test performance; reducing the area of corneal staining with fluorescein; increase in the height of the tear meniscus. We also studied the total antioxidant activity (AOA) of the tear fluid of patients, which was assessed by recording the kinetics of chemiluminescence in the hemoglobin - luminol - hydrogen peroxide system. The water-soluble equivalent of tocopherol, Trolox, was used as a standard. AOA was expressed in μmol of Trolox [15].
The safety and tolerability of the drugs were assessed based on the results of a clinical examination, including subjective complaints of the patient, allergic reactions and objective phenomena of intolerance.
Results. As a result of treatment, visual acuity increased slightly in the Visomitin group and remained virtually unchanged in the Natural Tear group (Fig. 1).
Picture 1 . Dynamics of visual acuity of patients during treatment with Visomitin and Natural Tear. Shown are the absolute values of visual acuity (A) and changes in this parameter as a result of treatment (B)
* — p<0.05; vertical bars - confidence interval
Upon presentation, all patients reported swelling and hyperemia of the eyelids and conjunctiva of varying severity.
The average score for swelling and hyperemia of the eyelids at the beginning of treatment in the Visomitin group was 1.12, and in the Natural Tears group - 1.5. During the treatment, on the 2nd and 3rd days, eyelid hyperemia remains quite stable in the two groups; by the third visit, the indicators of this symptom are compared (0.9 points), then in the Visomitin group, eyelid hyperemia begins to disappear more quickly than in group Natural Tears, and by the final visit these indicators correspond to: Visomitin - 0.25 points; Natural tear - 0.5 points.
Before starting treatment, all patients showed clinical signs of changes in the conjunctiva of varying severity. Conjunctival hyperemia was registered in the Visomitin group at a level of 1.65 points and in the Natural Tears group - 1.6 points. Improvement in the clinical condition of the conjunctiva occurs quite evenly in the two groups from the 2nd to the 7th day of treatment, and by the 21st day there is a more pronounced decrease in this symptom - the average indicator in the Visomitin group is 0.1±0.3 points, and in natural tears group - 0.7±0.7 points. Thus, after 3 weeks of treatment, Visomitin almost completely relieves conjunctival hyperemia, while Natural Tear only reduces this indicator by half.
Swelling of the bulbar conjunctiva was recorded in all patients and was 1.65 and 1.9 points, respectively, in the Visomitin and Natural Tear groups. These changes gradually fade away in the Visomitin group and hardly change in the Natural Tears group (0.9 and 1.7 points, respectively).
The condition of the cornea was considered according to several criteria . Biomicroscopy with a cobalt filter assessed the stability of the precorneal tear film (PTF) using the Norn test and changes in the epithelium (staining of xerotic cells with fluorescein). The average Norn test on the day of treatment was 2.1 seconds. in the Visomitin group and 2.5 sec. in the group Natural Tears. By the final visit, there was a trend towards an increase in PSP stability, and this trend was more pronounced in the Visomitin group (Fig. 2).
Figure 2. Dynamics of the Norn test in patients during treatment with Visomitin and Natural Tear. Absolute values (A), and changes during treatment (B). Vertical bars - confidence interval
Changes in the epithelium of the conjunctiva and cornea were assessed by the prevalence of microerosions when stained with fluorescein. The area of the lesion was assessed in points (maximum scale 9 points) and at the beginning of the study averaged 1.8 points in the Visomitin group and 0.9 points in the Natural Tears group. Despite such unequal initial data, keratopathy in the Natural Tears group remained within 0.9 points for four visits, while in the Visomitin group the reduction in the number of microerosions proceeded quite quickly and by the final visit was 0.07 points (almost complete cure ) versus 0.3 points for the Natural Tears group (Fig. 3).
Figure 3. Dynamics of corneal changes in patients treated with Visomitin and Natural Tear
Before the start of treatment, higher average tear meniscus heights were recorded in the Natural Tears group. At the final visit, this indicator in the Visomitin group increased by 206%, and in the Natural Tears group - by 152%.
Tear production indicators were assessed using the Schirmer test. Before treatment, this indicator in the Visomitin group averaged 2.8 mm, and in the Natural Tears group - 3.5 mm. During the treatment, its gradual increase was noted in the two groups, and by the final visit, the Schirmer test values were 5 mm in both the Visomitin group and the Natural Tear group. However, noteworthy is the fact that at the end of the course of therapy in the Visomitin group there were no patients with extremely low test values (Fig. 4).
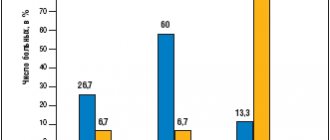
Figure 4. Proportion of eyes (in %) with an extremely low Schirmer test value (less than 2 mm) during treatment with Visomitin and Natural Tear
* — p<0.05; vertical bars - confidence interval
Before treatment, the level of antioxidant activity (AOA) in the group receiving Visomitin was 1920±169 µM Trolox, in the group receiving Natural Tear - 2205±130 µM Trolox. After a course of treatment lasting 21 days, the AOA level was 2210±191 µM Trolox and 2167±196 µM Trolox, respectively. No significant differences were found either between the groups or compared with the baseline. According to the present study, it was not possible to identify a correlation between the level of tear AOA and the clinical manifestations of HS. However, we found that in some patients, during treatment, AOA increased compared to the initial level, in others it decreased or did not change (the change did not exceed 20%). Depending on the change in AOA, we divided each group into 3 subgroups. In both groups there were almost the same number of eyes (13.7 and 12.5%) in which AOA did not change. An increase in AOA was observed in 18.8% of eyes in the group of patients receiving Visomitin, and in only 10% of eyes in the group receiving Natural Tear. A decrease in AOA was noted in 13.7% of eyes in the group of patients receiving Visomitin, while in the group receiving Natural Tear, such eyes were 25%.
Inflammation in FH can affect tear AOA in different ways. On the one hand, inflammation promotes the consumption of endogenous antioxidants, on the other hand, due to increased capillary permeability, plasma antioxidants more actively penetrate into tears. Therefore, during treatment, both an increase and a decrease in AOA is possible. It should be noted that an increase in AOA was observed in individuals who had a low initial AOA level, and a decrease in those who had it above the group average. Over 3 weeks of treatment, the number of patients who did not express complaints about the presence of signs of dry eye syndrome increased from 0 to 60% in the Visomitin group and only by 20% in the Natural Tear group (Fig. 5).
Figure 5. Proportion of patients (in%) who did not express complaints during treatment with Visomitin and Natural Tear
* — p<0.001; vertical bars - confidence interval
Discussion: a comparative analysis of the results of treatment of 40 patients with dry eye syndrome in two equal groups treated with Visomitin and Natural Tear indicates the following: the use of Visomitin as a medicinal product is more effective than the use of Natural Tear, this is manifested by an earlier and more pronounced normalization of the condition of the conjunctiva , epithelization of the cornea and the formation of a more stable epithelial cover. Also in this group there was a better dynamics of increasing the stability of PSP than in the control group. Indicators of the Schirmer test in the Visomitin group increased by 80% during treatment, and in the Natural Tears group - by 40%. According to the Schirmer test indicators, the proportion of eyes with extremely low values (less than 2 mm) predominated in the Visomitin group, and by the end of treatment, this severe category of patients remained only in the Natural Tears group. In patients with an extremely low initial Norn test value who were treated with Visomitin, its increase occurred earlier than in patients with a similar test value who received Natural Tear. Tolerability of the drug was assessed as “Very good” in the Visomitin group in 100% of patients, and in the Natural Tears group, tolerability in 40% of patients and “Very good” in 60% of patients.
Summarizing the data, we can draw the following conclusions:
1. Visomitin is well tolerated by patients, without causing discomfort or subjective discomfort. During the observation period, not a single undesirable phenomenon was noted, either from the conjunctiva or from the general side.
2. The use of Visomitin in the treatment of dry eye syndrome gives a more pronounced and stable therapeutic effect than the use of the drug Natural Tear. At the same time, the greatest effect was observed between 14 and 21 days. It can be expected that continuation of treatment beyond three weeks will have even better results.
3. Visomitin caused an increase in AOA in twice as many eyes as natural tears; There were 18.8% of such eyes, and the average increase in AOA was 40.4%.
4. An increase in AOA was observed in patients with a low initial AOA level, and a decrease in those with a high AOA level.
5. Tolerability of the drug was rated as “Very good” in the Visomitin group in 100% of patients, and in the Natural Tears group, tolerability in 40% of patients and “Very good” in 60% of patients.
E.V. Yani, L.A. Katargina, N.B. Chesnokova, O.V. Beznos, A.Yu. Savchenko, V.A. Vygodin, E.Yu. Gudkova, A.A. Zamyatnin (Jr.), M.V. Skulachev
Research Institute of Eye Diseases named after. Helmholtz, Moscow
First Moscow State Medical University named after. THEM. Sechenov
Moscow State University named after. M.V. Lomonosov
Mitotech LLC
Zamyatnin Andrey Aleksandrovich - candidate of biological sciences, senior researcher. Department of Cell Signaling Systems Research Institute of Physics and Biology named after. A.N. Belozersky Moscow State University. M.V. Lomonosov
Literature:
1. Brzhesky V.V., Somov E.E. Corneal-conjunctival xerosis (diagnosis, clinical picture, treatment). — Ed. 2nd, part. reworked and additional - St. Petersburg: Levsha, 2003. - 119 p.
2. Maitchouk DY, Beuerman RW, Ohta T. et al. Tear production after unilateral removal of main lacrimal gland in squirrel monkeys // Archives of Ophthalmology. - 2000. - No. 2. - P. 246-253.
3. Brzhesky V.V., Somov E.E. Modern methods for diagnosing dry eye syndrome. — Dry eye syndrome // Special. publication of the Moscow Association of Ophthalmol. - 2002. - No. 2. - P. 3-8.
4. Fox RI Systemic diseases associated with dry eye // Intern. Ophthalmol. Clin. - 1994. - Vol. 34, No. 1. - P. 71-78;
5. Moss SE, Klein R., Klein BE Incidence of dry eye in an older population // Arch. Ophthalmol. - 2004. - Vol. 122, No. 3. - P. 369-373.
6. Astakhov S.Yu., Astakhov Yu.S., Tkachenko N.V. Changes in the conjunctiva and cornea in patients with glaucoma during local antihypertensive therapy // Glaucoma News. - 2010. - No. 1. - P. 16-18;
7. Maychuk D.Yu. Clinical forms of secondary dry eye in ophthalmic surgery and therapy // Eye World. - 2002. - No. 3. - P. 36-37.
8. Schaumberg DA, Buring JE, Sullivan DA et al. Hormone replacement therapy and dry eye syndrome // JAMA. - 2001, No. 286. - R. 2114-2119.
9. Zorov DB, Filburn CR, Klotz LO et al. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes // J. Exp. Med. - 2000 - V. 192(7) - P. 1001-1014.
10. Kurenkov V.V., Sheludchenko V.M., Maychuk D.Yu. The use of carnosine as an antioxidant agent in pharmacotherapy during excimer laser surgery. // Current issues of ophthalmology: materials of the conference. - Moscow. - 2000. - pp. 137-138.
11. Liberman EA, Topaly VP, Tsofina LM et al. Mechanism of coupling of oxidative phosphorylation and the membrane potential of mitochondria // Nature. - 1969. - V. 222(5198). - P. 1076-1078.
12. Antonenko Yu.N., Avetisyan A.V., Bakeeva L.E. etc. A plastoquinone derivative targeted to mitochondria as a means of interrupting the aging program. Cationic derivatives of plastoquinone: synthesis and in vitro research // Biochemistry. - 2008. - T. 73(12). - pp. 1589-1606.
13. Arkhipova L.T., Arkhipova M.M., Bakeeva L.E. etc. A plastoquinone derivative targeted to mitochondria as a means of interrupting the aging program. Age-related eye diseases. SkQ restores vision to blind animals // Biochemistry. - 2008. - T. 73(12). — S. 1641-1654.
14. Skulachev MV, Antonenko YN, Anisimov VN et al. Mitochondrial-targeted plastoquinone derivatives. Effect on senescence and acute age-related pathologies // Current Drug Targets. - 2011. - V. 12(6). — P. 800-826.
15. Gulidova O.V., Lyubitsky O.B., Klebanov G.I. and others. Changes in the antioxidant activity of tear fluid in experimental burn eye disease // Bulletin. exp. biol. and medicine. - 1999. - T. 128, No. 11. - P. 571-574.
Vizomitin price
The average price of Visomitin eye drops in Russia is 480-595 rubles. Buying Visomitin in St. Petersburg will cost 465-760 rubles, in Novosibirsk - 445-480 rubles, in Yekaterinburg - 492-655 rubles.
Where to buy Visomitin in Moscow?
Almost all pharmacies in Moscow constantly have this drug in stock, the price range is quite large - from 440 to 620 rubles.
The price of academician Skulachev’s eye drops in Ukraine is an average of 380 hryvnia.
- Online pharmacies in RussiaRussia
First experience of using the drug "Visomitin" in the treatment of "dry eye"
The article presents the results of an open randomized controlled comparative clinical study of the effectiveness and safety of the drug "Visomitin" in the treatment of patients with dry eye syndrome. Visomitin is the first drug with a mitochondria-targeted antioxidant as the main active ingredient. Visomitin has shown high effectiveness in the treatment of dry eye, which is confirmed by an improvement in subjective sensations, changes in visual acuity, normalization of the clinical picture, and improvement in diagnostic test parameters.
The first experience of using the drug Vizomitin in the treatment of “dry eyes”
Results on open randomized controlled comparative clinical trial on efficacy and safety of novel pharmaceutical Visomitin in the treatment of 'dry eye' patients are reported. Visomitin is the first pharmaceutical containing mitochondria-targeted antioxidant as an active substance. In this study Visomitin showed high efficacy in dry eye treatment. Results on recovery of subjective condition, visual acuity, clinical parameters and diagnostic tests data are reported.
The problem of dry eye syndrome (DES) has recently been widely covered in the ophthalmological literature. This disease combines a large number of pathological changes in the ocular surface and occurs in numerous patients not only with ophthalmological problems [1]. According to research conducted by the Johns Hopkins University International Task Force, DES occurs in 11% of people aged 30-60 years and in 15% over 65 years [2]. According to Russian authors, among patients over 50 years of age, dry eye syndrome occurs in more than 67% of cases [3]. Concomitant somatic pathology in old age is itself the cause of the manifestations of dry eye syndrome; the need to take various medications (such as antihypertensives, adrenergic agonists, antiarrhythmic drugs, antiparkinsonian drugs, etc.) also leads to a decrease in tear production [4, 5]. The most important aspect of the development of dry eye disease is concomitant ocular pathology, such as glaucoma and cataracts. The use of antihypertensive eye drops worsens the condition of the anterior part of the eye and aggravates the course of dry eye syndrome [6]. Modern capabilities of cataract surgery make it possible to perform operations in the early stages of the development of lens opacities, but in the postoperative period there are often problems with impaired tear production and, as a consequence, a decrease in the quality of life [7]. A certain proportion of patients with dry eye syndrome are pre- and menopausal women, which is associated with hormonal changes in the body [8].
Considering the above, it is obvious that with age there is an increase in the number of patients with dry eye syndrome. In addition, oxidative stress—damage to molecules, cells, and tissues by free radicals—plays a key role in the pathogenesis of dry eye syndrome. The tissues of the eye are most vulnerable to attack by free radicals and reactive oxygen species (ROS). One of the most powerful producers of endogenous ROS in our cells under stress conditions are mitochondria. Such ROS damage mitochondria, disrupting the functioning of the electron transport chain, which leads to an even greater increase in ROS production due to the reduction of molecular oxygen in the initial and middle links of this chain [9]. Thus, mitochondria are involved in a kind of “vicious circle” of oxidative stress in the cell.
At the same time, antioxidant drugs have not yet taken their rightful place in the treatment of dry eye syndrome and the main attention is still paid to tear replacement therapy. A method to combat oxidative damage was proposed back in the 60s, when the use of antioxidants began [10]. However, due to the presence of membranes, antioxidants are virtually unable to penetrate mitochondria and neutralize endogenous ROS produced in the electron transport chain. It was proposed to combine the antioxidant with the lipophilic cation alkyltriphenylphosphonium, which, due to its positive charge and ability to pass through biological membranes, was shown [11] to specifically accumulate in the mitochondria of living cells. The resulting substance, named SkQ1 (INN - plastoquinonyldecyltriphenylphosphonium bromide), specifically accumulates in mitochondria, exhibits high antioxidant activity and is a regenerated antioxidant, because its spent (oxidized) form is reduced by the electron transport chain in vivo [12]. Preclinical studies have shown the effectiveness of this substance in models of a number of diseases [13, 14]. Plastoquinonyldecyltriphenylphosphonium bromide is the active substance of Visomitin eye drops, the world's first registered drug with a mitochondria-targeted antioxidant as the main active ingredient.
The purpose of the work was to study the effectiveness of using a new keratoprotective drug for the treatment of dry eye syndrome - Visomitin (registration number LP-001355), the world's first registered drug with a mitochondria-targeted antioxidant as the main active ingredient.
Materials and methods: a comparative study was conducted in which 40 patients took part, including 35 women and 5 men; 20 patients were treated with the drug “Visomitin” and 20 with the drug “Natural Tear” (the commercial name of a tear replacement drug produced for the treatment of dry eye syndrome by Alcon and which is one of the most popular drugs prescribed for this disease). The average age of the Visomitin group was 54.8±0.12 years, in the Natural Tears group - 53.8±0.15 years. All patients contacted the Institute with complaints and clinical presentations characteristic of DES. The first group was prescribed Visomitin in the form of instillations into the conjunctival cavity of the eye, 1 drop 3 times a day. Patients of the second group received eye drops “Natural Tear”, 1 drop 3 times a day. The course of treatment is 21 days with control visits on the 2nd, 3rd, 7th, 14th and 21st days of treatment.
Evaluation of the effectiveness of treatment was carried out according to the following criteria: dynamics of the functional state of the organ of vision and visual acuity indicators; dynamics of the Norn test; improvement of Schirmer test performance; reducing the area of corneal staining with fluorescein; increase in the height of the tear meniscus. We also studied the total antioxidant activity (AOA) of the tear fluid of patients, which was assessed by recording the kinetics of chemiluminescence in the hemoglobin - luminol - hydrogen peroxide system. The water-soluble equivalent of tocopherol, Trolox, was used as a standard. AOA was expressed in μmol of Trolox [15].
The safety and tolerability of the drugs were assessed based on the results of a clinical examination, including subjective complaints of the patient, allergic reactions and objective phenomena of intolerance.
Results. As a result of treatment, visual acuity increased slightly in the Visomitin group and remained virtually unchanged in the Natural Tear group (Fig. 1).
Picture 1 . Dynamics of visual acuity of patients during treatment with Visomitin and Natural Tear. Shown are the absolute values of visual acuity (A) and changes in this parameter as a result of treatment (B)
* — p<0.05; vertical bars - confidence interval
Upon presentation, all patients reported swelling and hyperemia of the eyelids and conjunctiva of varying severity.
The average score for swelling and hyperemia of the eyelids at the beginning of treatment in the Visomitin group was 1.12, and in the Natural Tears group - 1.5. During the treatment, on the 2nd and 3rd days, eyelid hyperemia remains quite stable in the two groups; by the third visit, the indicators of this symptom are compared (0.9 points), then in the Visomitin group, eyelid hyperemia begins to disappear more quickly than in group Natural Tears, and by the final visit these indicators correspond to: Visomitin - 0.25 points; Natural tear - 0.5 points.
Before starting treatment, all patients showed clinical signs of changes in the conjunctiva of varying severity. Conjunctival hyperemia was registered in the Visomitin group at a level of 1.65 points and in the Natural Tears group - 1.6 points. Improvement in the clinical condition of the conjunctiva occurs quite evenly in the two groups from the 2nd to the 7th day of treatment, and by the 21st day there is a more pronounced decrease in this symptom - the average indicator in the Visomitin group is 0.1±0.3 points, and in natural tears group - 0.7±0.7 points. Thus, after 3 weeks of treatment, Visomitin almost completely relieves conjunctival hyperemia, while Natural Tear only reduces this indicator by half.
Swelling of the bulbar conjunctiva was recorded in all patients and was 1.65 and 1.9 points, respectively, in the Visomitin and Natural Tear groups. These changes gradually fade away in the Visomitin group and hardly change in the Natural Tears group (0.9 and 1.7 points, respectively).
The condition of the cornea was considered according to several criteria . Biomicroscopy with a cobalt filter assessed the stability of the precorneal tear film (PTF) using the Norn test and changes in the epithelium (staining of xerotic cells with fluorescein). The average Norn test on the day of treatment was 2.1 seconds. in the Visomitin group and 2.5 sec. in the group Natural Tears. By the final visit, there was a trend towards an increase in PSP stability, and this trend was more pronounced in the Visomitin group (Fig. 2).
Figure 2. Dynamics of the Norn test in patients during treatment with Visomitin and Natural Tear. Absolute values (A), and changes during treatment (B). Vertical bars - confidence interval
Changes in the epithelium of the conjunctiva and cornea were assessed by the prevalence of microerosions when stained with fluorescein. The area of the lesion was assessed in points (maximum scale 9 points) and at the beginning of the study averaged 1.8 points in the Visomitin group and 0.9 points in the Natural Tears group. Despite such unequal initial data, keratopathy in the Natural Tears group remained within 0.9 points for four visits, while in the Visomitin group the reduction in the number of microerosions proceeded quite quickly and by the final visit was 0.07 points (almost complete cure ) versus 0.3 points for the Natural Tears group (Fig. 3).
Figure 3. Dynamics of corneal changes in patients treated with Visomitin and Natural Tear
Before the start of treatment, higher average tear meniscus heights were recorded in the Natural Tears group. At the final visit, this indicator in the Visomitin group increased by 206%, and in the Natural Tears group - by 152%.
Tear production indicators were assessed using the Schirmer test. Before treatment, this indicator in the Visomitin group averaged 2.8 mm, and in the Natural Tears group - 3.5 mm. During the treatment, its gradual increase was noted in the two groups, and by the final visit, the Schirmer test values were 5 mm in both the Visomitin group and the Natural Tear group. However, noteworthy is the fact that at the end of the course of therapy in the Visomitin group there were no patients with extremely low test values (Fig. 4).
Figure 4. Proportion of eyes (in %) with an extremely low Schirmer test value (less than 2 mm) during treatment with Visomitin and Natural Tear
* — p<0.05; vertical bars - confidence interval
Before treatment, the level of antioxidant activity (AOA) in the group receiving Visomitin was 1920±169 µM Trolox, in the group receiving Natural Tear - 2205±130 µM Trolox. After a course of treatment lasting 21 days, the AOA level was 2210±191 µM Trolox and 2167±196 µM Trolox, respectively. No significant differences were found either between the groups or compared with the baseline. According to the present study, it was not possible to identify a correlation between the level of tear AOA and the clinical manifestations of HS. However, we found that in some patients, during treatment, AOA increased compared to the initial level, in others it decreased or did not change (the change did not exceed 20%). Depending on the change in AOA, we divided each group into 3 subgroups. In both groups there were almost the same number of eyes (13.7 and 12.5%) in which AOA did not change. An increase in AOA was observed in 18.8% of eyes in the group of patients receiving Visomitin, and in only 10% of eyes in the group receiving Natural Tear. A decrease in AOA was noted in 13.7% of eyes in the group of patients receiving Visomitin, while in the group receiving Natural Tear, such eyes were 25%.
Inflammation in FH can affect tear AOA in different ways. On the one hand, inflammation promotes the consumption of endogenous antioxidants, on the other hand, due to increased capillary permeability, plasma antioxidants more actively penetrate into tears. Therefore, during treatment, both an increase and a decrease in AOA is possible. It should be noted that an increase in AOA was observed in individuals who had a low initial AOA level, and a decrease in those who had it above the group average. Over 3 weeks of treatment, the number of patients who did not express complaints about the presence of signs of dry eye syndrome increased from 0 to 60% in the Visomitin group and only by 20% in the Natural Tear group (Fig. 5).
Figure 5. Proportion of patients (in%) who did not express complaints during treatment with Visomitin and Natural Tear
* — p<0.001; vertical bars - confidence interval
Discussion: a comparative analysis of the results of treatment of 40 patients with dry eye syndrome in two equal groups treated with Visomitin and Natural Tear indicates the following: the use of Visomitin as a medicinal product is more effective than the use of Natural Tear, this is manifested by an earlier and more pronounced normalization of the condition of the conjunctiva , epithelization of the cornea and the formation of a more stable epithelial cover. Also in this group there was a better dynamics of increasing the stability of PSP than in the control group. Indicators of the Schirmer test in the Visomitin group increased by 80% during treatment, and in the Natural Tears group - by 40%. According to the Schirmer test indicators, the proportion of eyes with extremely low values (less than 2 mm) predominated in the Visomitin group, and by the end of treatment, this severe category of patients remained only in the Natural Tears group. In patients with an extremely low initial Norn test value who were treated with Visomitin, its increase occurred earlier than in patients with a similar test value who received Natural Tear. Tolerability of the drug was assessed as “Very good” in the Visomitin group in 100% of patients, and in the Natural Tears group, tolerability in 40% of patients and “Very good” in 60% of patients.
Summarizing the data, we can draw the following conclusions:
1. Visomitin is well tolerated by patients, without causing discomfort or subjective discomfort. During the observation period, not a single undesirable phenomenon was noted, either from the conjunctiva or from the general side.
2. The use of Visomitin in the treatment of dry eye syndrome gives a more pronounced and stable therapeutic effect than the use of the drug Natural Tear. At the same time, the greatest effect was observed between 14 and 21 days. It can be expected that continuation of treatment beyond three weeks will have even better results.
3. Visomitin caused an increase in AOA in twice as many eyes as natural tears; There were 18.8% of such eyes, and the average increase in AOA was 40.4%.
4. An increase in AOA was observed in patients with a low initial AOA level, and a decrease in those with a high AOA level.
5. Tolerability of the drug was rated as “Very good” in the Visomitin group in 100% of patients, and in the Natural Tears group, tolerability in 40% of patients and “Very good” in 60% of patients.
E.V. Yani, L.A. Katargina, N.B. Chesnokova, O.V. Beznos, A.Yu. Savchenko, V.A. Vygodin, E.Yu. Gudkova, A.A. Zamyatnin (Jr.), M.V. Skulachev
Research Institute of Eye Diseases named after. Helmholtz, Moscow
First Moscow State Medical University named after. THEM. Sechenov
Moscow State University named after. M.V. Lomonosov
Mitotech LLC
Zamyatnin Andrey Aleksandrovich - candidate of biological sciences, senior researcher. Department of Cell Signaling Systems Research Institute of Physics and Biology named after. A.N. Belozersky Moscow State University. M.V. Lomonosov
Literature:
1. Brzhesky V.V., Somov E.E. Corneal-conjunctival xerosis (diagnosis, clinical picture, treatment). — Ed. 2nd, part. reworked and additional - St. Petersburg: Levsha, 2003. - 119 p.
2. Maitchouk DY, Beuerman RW, Ohta T. et al. Tear production after unilateral removal of main lacrimal gland in squirrel monkeys // Archives of Ophthalmology. - 2000. - No. 2. - P. 246-253.
3. Brzhesky V.V., Somov E.E. Modern methods for diagnosing dry eye syndrome. — Dry eye syndrome // Special. publication of the Moscow Association of Ophthalmol. - 2002. - No. 2. - P. 3-8.
4. Fox RI Systemic diseases associated with dry eye // Intern. Ophthalmol. Clin. - 1994. - Vol. 34, No. 1. - P. 71-78;
5. Moss SE, Klein R., Klein BE Incidence of dry eye in an older population // Arch. Ophthalmol. - 2004. - Vol. 122, No. 3. - P. 369-373.
6. Astakhov S.Yu., Astakhov Yu.S., Tkachenko N.V. Changes in the conjunctiva and cornea in patients with glaucoma during local antihypertensive therapy // Glaucoma News. - 2010. - No. 1. - P. 16-18;
7. Maychuk D.Yu. Clinical forms of secondary dry eye in ophthalmic surgery and therapy // Eye World. - 2002. - No. 3. - P. 36-37.
8. Schaumberg DA, Buring JE, Sullivan DA et al. Hormone replacement therapy and dry eye syndrome // JAMA. - 2001, No. 286. - R. 2114-2119.
9. Zorov DB, Filburn CR, Klotz LO et al. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes // J. Exp. Med. - 2000 - V. 192(7) - P. 1001-1014.
10. Kurenkov V.V., Sheludchenko V.M., Maychuk D.Yu. The use of carnosine as an antioxidant agent in pharmacotherapy during excimer laser surgery. // Current issues of ophthalmology: materials of the conference. - Moscow. - 2000. - pp. 137-138.
11. Liberman EA, Topaly VP, Tsofina LM et al. Mechanism of coupling of oxidative phosphorylation and the membrane potential of mitochondria // Nature. - 1969. - V. 222(5198). - P. 1076-1078.
12. Antonenko Yu.N., Avetisyan A.V., Bakeeva L.E. etc. A plastoquinone derivative targeted to mitochondria as a means of interrupting the aging program. Cationic derivatives of plastoquinone: synthesis and in vitro research // Biochemistry. - 2008. - T. 73(12). - pp. 1589-1606.
13. Arkhipova L.T., Arkhipova M.M., Bakeeva L.E. etc. A plastoquinone derivative targeted to mitochondria as a means of interrupting the aging program. Age-related eye diseases. SkQ restores vision to blind animals // Biochemistry. - 2008. - T. 73(12). — S. 1641-1654.
14. Skulachev MV, Antonenko YN, Anisimov VN et al. Mitochondrial-targeted plastoquinone derivatives. Effect on senescence and acute age-related pathologies // Current Drug Targets. - 2011. - V. 12(6). — P. 800-826.
15. Gulidova O.V., Lyubitsky O.B., Klebanov G.I. and others. Changes in the antioxidant activity of tear fluid in experimental burn eye disease // Bulletin. exp. biol. and medicine. - 1999. - T. 128, No. 11. - P. 571-574.