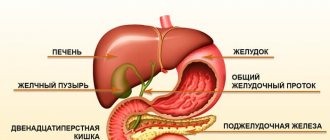
To do or not to do, to take or not to take medicine, to take a test or not to take a test. We are always faced with a choice and are often afraid of making a mistake. Iron can be said that you can’t go wrong with iron.
The adult human body contains about 3–4.5 g of iron. More than half of the iron is represented by heme in the composition of hemoglobin, a third is stored in the depot in the form of ferritin and hemosiderin. Moreover, iron deficiency is the most common deficiency condition worldwide, silently leading to anemia.
The main causes of iron deficiency:
Reduced intake of iron into the body due to impaired absorption due to diseases of the gastrointestinal tract, fasting, refusal, or reduced consumption of foods containing iron.
Increased iron loss during chronic, repeated blood loss (uterine, gastric, intestinal, etc.), as well as during acute massive bleeding.
Increased consumption of iron by the body in children during periods of growth, in women during pregnancy and subsequent breastfeeding of the child.
Clinical pharmacology of modern iron preparations and their place in the treatment of iron deficiency anemia
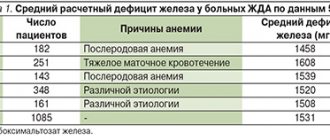
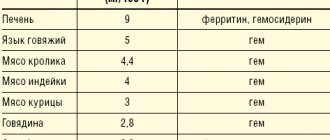
Latent iron deficiency in the population of Europe and Russia is 30–40%, and in some regions (North, Northern Caucasus, Eastern Siberia) – 50–60% [2,3]. Iron deficiency anemia is a hypochromic microcytic anemia that occurs as a result of a decrease in iron content in the body and is an independent nosological form (code D-50 according to ICD-10). Latent iron deficiency is considered a functional disorder, does not have an independent code and is usually coded in the section “Insufficiency of other nutrients” (E61). Chronic IDA is a consequence of a long-term negative iron balance in the body. The reasons for its development may be chronic blood loss due to gastrointestinal bleeding (erosive gastritis, peptic ulcer, hemorrhoids, taking acetylsalicylic acid, indomethacin), uterine bleeding during heavy menstruation, as well as tumors (rectal and colon cancer, bladder cancer), donation, increased need for iron during pregnancy and lactation, in adolescence (juvenile chlorosis), with parasitic diseases (diphyllobothriasis), insufficient iron intake (gastroduodenitis, colitis, gastrectomy), nutritional iron deficiency in vegetarians [2,4]. Pathogenesis. The total amount of iron in the body is 4–4.5 g. Iron is involved in the functioning of all biological systems, being an obligatory and indispensable component of various proteins and enzymes that provide the necessary level of systemic and cellular aerobic metabolism, as well as redox homeostasis in the body as a whole . Iron plays an important role in maintaining a high level of immune resistance; its adequate content in the body contributes to the full functioning of nonspecific defense factors, cellular and local immunity. Normal iron content is necessary for complete phagocytosis, synthesis of lysozyme, interferon, ensures high activity of natural killer cells and the bactericidal ability of serum [5,6]. The daily requirement for iron is 10 mg for men, 18 mg for women (during pregnancy – 38 mg, lactation – 33 mg) [6]. The need for iron is also increased in active donors. In children and adolescents, due to the increased needs of the growing body and insufficient intake of iron from food, the iron balance is often disturbed [7]. The main source of iron for humans is food products of animal origin, which contain iron in the most digestible form (as heme). The absorption of iron from foods decreases after heat treatment, freezing, and long-term storage. Iron, entering the body with food, is absorbed in the intestine (most intensively in the duodenum and the initial part of the jejunum). Iron absorption depends on the nature of the food, the calorie content of the diet and the absorption capacity of the small intestine. Iron in heme is absorbed much better. People who eat meat get more heme iron (in myoglobin) than vegetarians. Strict vegetarians may develop iron deficiency over time because vegetables and grains contain substances that interfere with iron absorption, particularly phosphates [7,8]. Fructose, hydrochloric, ascorbic, succinic, pyruvic acids, cysteine, sorbitol and alcohol enhance iron resorption. Ferrous inorganic iron is absorbed much better than oxide iron contained in meat products [9]. From the gastrointestinal tract (GIT), iron is absorbed only in the divalent state, the conversion to which is ensured by organic acids, in particular ascorbic acid. The main share of absorbed iron in the transport protein, transferrin, is transferred to the bone marrow, where it is used for the synthesis of heme-containing compounds (hemoglobin, myoglobin, enzymes), non-heme enzymes (for example, NADH dehydrogenase), and metalloproteins. In tissues, iron is deposited in the form of ferritin and hemosiderin, with predominant deposition in the liver, spleen and muscles. Iron deficiency in the body develops when iron losses exceed 2 mg/day. [9]. However, not every iron deficiency is accompanied by anemia; prelatent and latent iron deficiency are also distinguished [2,5]. Prelatent deficiency develops when the intake of iron from food does not meet physiological needs (body growth, menstruation, pregnancy), but the insufficient intake of iron is covered by its reserves. At the same time, iron reserves are depleted. Latent deficiency is the next stage at which the supply of iron to the cells of the erythroid germ is reduced and the production of red blood cells is limited. IDA with hypochromia and microcytosis develops as a result of a long-term negative iron balance, when hemoglobin synthesis decreases. Most often, anemic syndrome associated with the development of IDA occurs in the practice of a general practitioner, general practitioner (family doctor), hematologist, obstetrician-gynecologist. According to currently accepted standards of higher professional and postgraduate professional education, these specialists are competent to suspect IDA syndrome based on the clinical picture and peripheral blood picture, develop a program of diagnostic and therapeutic measures, justify a program of preventive measures in patients with IDA, taking into account the presence of factors the risk of relapse. The clinical picture of IDA depends on the stage of iron deficiency. Stages of iron deficiency (ID): • pre-latent iron – characterized by a decrease in iron reserves, but without a decrease in the amount spent on erythropoiesis (reserve iron deficiency); • latent iron deficiency – characterized by complete depletion of iron stores in the depot, a decrease in the level of ferritin in the blood serum, an increase in the total iron-binding capacity of the serum (TIBC) and the level of transferrin, but still without signs of the development of anemia (transport iron deficiency); • IDA is the final stage of iron deficiency, which occurs when the hemoglobin pool of iron decreases and is manifested by symptoms of anemia and hyposiderosis (obvious iron deficiency). There is a classic clinical symptomatology of IDA in the body, consisting of general symptoms of anemia caused by hemic hypoxia, as well as signs of tissue iron deficiency (sideropenic syndrome) (Table 1). Anemia that develops during pregnancy has an adverse effect on the intrauterine state of the fetus, contributing to the occurrence of fetal growth retardation syndrome and complications of the early neonatal period. In children during the newborn period, there is a large loss of body weight and a slower recovery, delayed loss of the umbilical cord remnant and delayed epithelization of the umbilical cord wound, and a long course of physiological jaundice [10,11]. Diagnostics. To diagnose iron deficiency in the body, indicators characterizing various funds of iron metabolism are determined: transport, functional, reserve and iron regulatory (Table 2). Laboratory signs that allow one to suspect and verify IDA are a low color index, a decrease in the MSI index (below 27 pg, normally 27–35 pg), hypochromia and microcytosis of erythrocytes, a decrease in serum iron levels, an increase in the TI value, a decrease in serum ferritin levels . Differential diagnosis for IDA is also carried out with anemia in chronic diseases, megaloblastic anemia associated with deficiency of vitamin B12 or folic acid, hemolytic and aplastic anemia (possible, among other things, due to the toxic effects of various classes of drugs, occupational hazards, such as lead, benzene) [ 12]. The differential diagnosis of IDA and anemia in chronic diseases is presented in Table 3. The most common causes of anemia in chronic diseases are chronic infections (diseases of the respiratory system, urinary tract, osteomyelitis), chronic non-infectious diseases (diffuse connective tissue diseases, rheumatic fever), malignant tumors ( cancer, lymphogranulomatosis, non-Hodgkin's lymphoma, leukemia). The hypochromic nature of anemia makes it very likely that it is iron deficiency (all IDA are hypochromic). However, the hypochromic nature of anemia does not exclude other pathogenetic variants of anemia (not all hypochromic anemias are iron deficiency). In this regard, differential diagnosis of IDA with other, less common hypochromic anemias (sideroachrestic anemia, thalassemia) is necessary. Determination of serum iron levels makes it possible to distinguish between these conditions at the stage of diagnostic search. Serum iron levels can be influenced by a number of factors: taking iron medications (vitamin preparations containing small amounts of iron, certain medications (oral contraceptives, allopurinol)), red blood cell transfusions, time of serum iron testing, thereby masking the true iron content in the serum , which should be taken into account when interpreting the results obtained. Treatment. In accordance with the etiological and pathogenetic factors of IDA, treatment should be comprehensive, aimed at eliminating the cause that caused the disease, and include adequate intake of microelements, vitamins, proteins and correction of iron deficiency. Iron is absorbed most effectively from foods in which it is contained in the form of heme, when it is actively captured and absorbed by the cells of the intestinal mucosa in unchanged form (beef tongue, rabbit, turkey, chicken, beef). The processes of heme absorption in the intestine do not depend on the acidity of the environment and inhibitory nutrients. As already mentioned, in cereals, fruits and vegetables, iron is in non-heme form, and absorption from them is much worse. The presence of oxalates, phosphates, tannin and other ferroabsorption inhibitors also contributes to a decrease in absorption (Table 4). It should be borne in mind that a complete and balanced diet in terms of the main ingredients allows only to “cover” the physiological need of the body for iron, but does not eliminate its deficiency, and should be considered as one of the auxiliary components of therapy. Currently, the indications for parenteral use of iron preparations have been narrowed: they are used in the presence of intestinal pathology with malabsorption (various enteritis, malabsorption syndrome, resection of the small intestine, Billroth II gastric resection with the formation of a “blind loop”). Parenteral iron supplements may be the treatment of choice if oral iron supplements are poorly tolerated. Currently, in the treatment of IDA, the oral use of combination drugs (containing both iron salts and other components), the leading of which is Ferro-Folgamma, seems promising. Ferro-Folgamma (Wörwag Pharma, Germany) is a multifactorial hematopoietic that includes all the necessary components (1 capsule contains 112.6 mg of iron sulfate (equivalent to 37 mg of iron ion), 5 mg of folic acid, 0.010 mg of cyanocobalamin), providing stimulation of the structural synthesis of Hb and increasing the reproduction of red blood cells by the red sprout of the bone marrow. The drug also contains ascorbic acid. The versatility of the drug is associated with its selective stimulating effect on the synthesis of the iron-containing and protein parts of hemoglobin. Ferrous sulfate has a high absorption rate in the gastrointestinal tract and practically does not form inaccessible complex compounds in it. The absorption coefficient is significantly enhanced by the presence of ascorbic acid in the preparation [12]. Sulfate replenishes iron deficiency in the body. Iron is part of hemoglobin, myoglobin and a number of enzymes. Being a structural component of heme, it takes part in erythropoiesis. Ascorbic acid helps improve the absorption of iron in the intestines. Cyanocobalamin (vitamin B12) and folic acid are involved in the formation and maturation of red blood cells. The active components of the Ferro-Folgamma drug are in a special neutral shell, which ensures their absorption, mainly from the upper part of the small intestine. The absence of local irritation on the gastric mucosa contributes to good tolerability of the drug in the gastrointestinal tract. Ferro-Folgamma is prescribed 1 capsule 3 times a day. The best effect is obtained by using the drug after meals. For mild anemia, it is recommended to take 1 capsule 3 times a day. within 3–4 weeks; for moderate forms – 1 capsule 3 times a day. within 8–12 weeks; in severe cases - 1 capsule 3 times a day. within 16 weeks. and more. The good clinical effect of the drug is confirmed by data from numerous clinical studies. Thus, in the studies of A.L. Vertkina et al. [14] have proven the high effectiveness of the drug Ferro-Folgamma (compared to other ferrodrugs) in the treatment of IDA. The authors observed 83 people (22 men and 61 women) aged from 17 to 92 years (average age – 57.7±1.4 years). In the group, the average hemoglobin level was reduced to 87.8±0.4 g/l, the average duration of anemia was 1.5±0.1 years. The causes of anemia were: acute or chronic blood loss - in 54.3% of cases, malabsorption - in 28.3%, other or combined causes - in 17.3% of cases. When using the drug Ferro-Folgamma, more pronounced positive dynamics of both clinical and laboratory parameters were observed compared with other ferrocontaining drugs [14] (Tables 5 and 6). The use of Ferro-Folgamma in the correction of IDA in chronic heart failure (CHF) has also been proven to be highly effective [15]. The authors selected for examination and treatment 42 patients with CHF combined with IDA (in the whole group Hb = 97.1 ± 3.7 g/l; serum Fe = 5.84 ± 0.51 mmol/l): in II FC HF – Hb = 102.3±3.6 g/l, with FC III HF – Hb = 97.6±3.3 g/l, with FC IV HF – Hb = 84.4±3.5 g/ l. During the treatment of CHF (ACE inhibitors, cardiac glycosides, diuretics), relief of anemic syndrome was carried out by prescribing Ferro-Folgamma (a complex of 100 mg of anhydrous Fe sulfate with 5 mg of folic acid, 10 μg of cyanocobalamin and 100 mg of ascorbic acid) in the total dose for the whole group –137.75±7.5 mg Fe. By the end of treatment (23.7±3 days), the hemoglobin level in general in the group of patients with CHF increased by 9.8%, serum Fe increased by 95.5%, exercise tolerance increased by 47.6%, ejection fraction ( EF) increased by 32.2%, stroke volume (SV) increased by 51.7%; Before treatment, 31 patients with CHF with IDA (73.8%) had HF class III–IV; after treatment, 23 patients (54.8%) switched to HF class I; 19 patients (45.2%) had class II HF. FC SN. Thus, all of the above suggests that correction of even a mild degree of anemia in patients with CHF can lead to a significant improvement in the pumping function of the heart and a decrease in the functional class of HF (NYHA). The use of Ferro-Folgamma in the treatment of anemia in pregnant women has also been proven to be highly effective. So, under the leadership of Professor V.A. Burlev and academician of the Russian Academy of Medical Sciences, professor V.N. Serov at the Federal State Institution “Scientific Center of Obstetrics, Gynecology and Perinatology named after. Academician V.I. Kulakov" conducted a number of studies on the problem of iron deficiency in pregnant women, postpartum women and gynecological patients [16–19]. The clinical effectiveness of the drug Ferro-Folgamma has been most fully studied, which has been successfully used for the treatment of latent iron deficiency (LDI) and manifest iron deficiency (MID) in obstetric and gynecological patients, both alone and in combination with recombinant erythropoietin preparations. The results obtained showed that the unique ratio between the optimal content of ferrous iron, folic acid and cyanocobalamin in one Ferro-Folgamma capsule allows one to achieve excellent results in the treatment of pregnant women, postpartum women and gynecological patients with not only mild, but also moderate and severe MID. The presence of folic acid and cyanocobalamin in the Ferro-Folgamma preparation is its additional advantage, since with MID in pregnant women there is often a deficiency of many vitamins, especially those involved in hematopoiesis. According to the data obtained, if there are indications for the use of recombinant erythropoietin, the optimal oral iron preparation for the combined treatment of MAD in women outside and during pregnancy, as well as after childbirth, is currently Ferro-Folgamma. The use of Ferro-Folgamma in pregnant women with anemia and when it is combined with gestosis leads to an improvement in the clinical condition, a significant increase in hematological (hemoglobin - by 15%, erythrocytes - by 10%, hematocrit - by 9%) and ferrokinetic parameters (serum iron - by 32%, ferritin – by 53%), folic acid level – by 24% and vitamin B12 – by 21.7% [18,19]. The effectiveness of using Ferro-Folgamma in the treatment of anemia in pregnant women was also proven in a study by V.A. Petrukhin and V.L. Grishin [19]. According to researchers, the use of Ferro-Folgamma 1 capsule 2 times a day. within 6 weeks. led to the normalization of clinical and laboratory indicators in all observed patients with the SDA syndrome developed during pregnancy [20]. Thus, in the treatment of UDA and latent iron deficiency, it is optimal to use the drug Ferro -folgamma, which allows you to achieve good and quick results. The presence of folic acid and vitamin B12 in the drug is its additional advantage, since in iron deficiency conditions there is often a deficiency of these vitamins. The presence of ascorbic acid improves the absorption and absorption of iron. The microcapsulated form of the drug excludes local irritation of the stomach and intestines. Scientific and practical experience convinces us of the appropriateness and high efficiency of use in therapeutic, hematological, obstetric and gynecological practice in the treatment of iron deficiency states of ferro -pholgamma as a drug with proven efficiency and non -punishment, which has the optimal ratio of cost and efficiency.
References 1. WHO. Official annual report. Geneva, 2002. 2. Zakharova N.O., Nikitin O.L. Iron deficiency anemia in elderly and senile patients: a scientific and practical guide for doctors. Samara, 2008. 60 p. 3. Gorodetsky V.V., Godulyan O.V. Iron deficiency conditions and iron deficiency anemia: treatment and diagnosis. M.: Medpraktika-M, 2004. pp. 1–28. 4. Dvoretsky L.I. Treatment of iron deficiency anemia // Russian Medical Journal. 2006. T. 6. No. 20. P. 1312–1316. 5. Kryukov N.N., Kachkovsky M.A., Verbovoy A.F., Babanov S.A. Therapist's handbook. Diagnostic reference book. M.: Astrel, 2012. 672 p. 6. Hillman R. Iron deficiency and other hyporegenerative anemias. Internal medicine according to Tinsley R. Harrisson // Difficult Patient. 2005. No. 2. P. 770. 7. Shekhtman M.M. Iron deficiency anemia and pregnancy // Ginekol. 2000. T. 2. No. 6. P. 164–171. 8. Serov V.N., Shapovalenko S.A., Flaks G.A. Diagnosis and treatment of iron deficiency in women at different periods of life // Atmosphere. Cardiology. 2008. No. 2. pp. 17–20. 9. Kosarev V.V., Babanov S.A., Verbovoy A.F. Handbook of clinical pharmacologist. Rostov-on-Don: Phoenix, 2011. 476 p. 10. Podzolkova N.M., Nesterova A.A., Nazarova S.V., Sheveleva T.V. Iron deficiency anemia in pregnant women // Russian Medical Journal. 2003. No. 11 (5). pp. 326–32. 11. Smirnova O.V., Chesnokova N.P., Mikhailov A.V. Iron deficiency anemia in pregnant women. Etiology and pathogenesis of metabolic and functional disorders. Saratov, 1994. 12. Kosarev V.V., Babanov S.A. Clinical pharmacology and rational pharmacotherapy. M.: Infra-M, 2012. 236 p. 13. Dvoretsky L.I., Zaspa E.A., Litvitsky P.F., Bolevich S.B., Menshova N.I. Free radical processes in patients with iron deficiency anemia during treatment with iron supplements // Therapeutic archive. 2006. No. 78 (1). pp. 52–57. 14. Vertkin A.L., Godulyan O.V., Gorodetsky V.V., Skotnikov A.S. Iron deficiency anemia and the choice of drug for its correction // Russian Medical Journal. 2010. No. 5. 15. Shilov A.M., Melnik M.V., Retivykh O.N., Kim I.R. Correction of iron deficiency anemia in chronic heart failure // Russian Medical Journal. 2005. No. 19. pp. 1254–1257. 16. Burlev, V.A., Konovodova E.N., Ordzhonikidze N.V., Serov V.N., Elokhina T.B., Ilyasova N.A. Treatment of latent iron deficiency and iron deficiency anemia in pregnant women // Russian Bulletin of Obstetrician-Gynecologist. 2006. No. 1. P. 64–68. 17. Konovodova E.N., Burlev V.A. Ferro-Folgamma + Erythropoietin - new possibilities for the treatment of anemia in patients with uterine fibroids // Farmateka. 2004. No. 15 (92). pp. 70–73. 18. Konovodova E.N., Burlev V.A., Kravchenko N.F., Karibdzhanov O.K., Murashko L.E., Sopoeva Zh.A. Transferrin saturation coefficient with iron in pregnant women // Problems of reproduction. 2002. No. 6. P. 45–47. 19. Murashko L.E., Konovodova E.N., Burlev V.A., Sopoeva Zh.A. Volumetric oxygen transport in pregnant women with anemia and gestosis during treatment with Ferro-Folgamma // Russian Medical Journal. 2002. T. 10. No. 7. pp. 364–367. 20. Petrukhin V.A., Grishin V.L. Treatment of anemia in pregnant women using the drug ferro-foil // Problems of reproduction. 2002. No. 6.
What tests show iron deficiency?
- Be sure to look at a general blood test with a leukocyte formula, since iron deficiency ultimately leads to the development of anemia. Iron deficiency anemia is indicated by a decrease in hemoglobin levels, an indicator of MCV (erythrocyte hypochromia). Often, the examination begins when there are symptoms of anemia (pallor, weakness, dizziness, loss of strength, decreased performance and attention, brittle nails and hair, etc.).
- Decreased serum iron concentration
- Increasing the total iron-binding capacity of serum
- Decreased transferrin saturation coefficient with iron
- Decreased serum ferritin concentration
It is recommended to take tests in the morning, from 8 to 11 am, since the level of iron in the serum is higher in the morning. They should also be taken in the absence of inflammation, which helps lower transferrin levels and increase ferritin levels.
The iron content in the serum is checked 5-7 days after taking vitamin-mineral complexes and dietary supplements containing iron, or short-term use of iron supplements.
Publications in the media
Acute poisoning with iron preparations is observed quite rarely; Due to the increasing popularity of drugs containing iron (for example, multivitamins with microelements, etc.), the frequency of poisoning has increased. Frequency. In 1988, more than 16,000 cases of intoxication with iron supplements were reported in the United States. The predominant age group is children (in 1984, 1,337 cases of iron poisoning out of 1,738 were registered among children under 6 years of age). Etiopathogenesis • Intoxication develops with oral intake of pure iron in a dose of more than 60 mg/kg • A lethal dose for humans is 200–250 mg/kg of pure iron (for 2-year-old children a lethal dose of pure iron is 3 g). A risk factor is children’s free access to iron supplements and iron-containing vitamins.
Clinical picture • Nausea, vomiting, diarrhea, drowsiness, pain in the upper abdomen, pallor, sweating. In severe cases - cyanosis, vomiting with blood, coagulopathy, acidosis, impaired microcirculation to the development of shock and coma. The first acute symptoms are often followed by a clear interval (apparent recovery) • After 12–48 hours, symptoms may recur; in severe cases, deep shock, severe acidosis, cyanosis, hyperthermia, convulsions, anuria develop; possible pulmonary edema, death • In the long term (2–6 weeks), stenosis develops in the pyloric or antrum of the stomach, cirrhosis of the liver and irreversible disorders of the central nervous system are possible. Laboratory tests • CBC • Determination of electrolytes and glucose in the blood • Determination of iron concentration in the blood serum, as well as TLC • Determination of the PT/MSR ratio (PT/international standardized ratio) and PTT • In case of severe poisoning - liver function tests. Special studies - radiography of the abdominal cavity and chest. Differential diagnosis - if there is no indication in the history of taking iron supplements, differential diagnosis should be carried out with gastritis, alcohol intoxication, viral infection, diabetic ketoacidosis, poisoning with other drugs.
TREATMENT Management tactics • In case of severe poisoning - hospitalization • Emetics, gastric lavage through a tube are carried out if the amount of pure iron taken by the patient exceeds 20 mg/kg, in the presence of characteristic symptoms • Specific (antidote) therapy • When taking a lethal dose of iron supplements - hemodialysis , peritoneal dialysis, exchange blood transfusions • Symptomatic therapy. Specific (antidote) therapy. If the iron content in plasma is more than 300 mg% - deferoxamine 1 g IV drip (at a rate of up to 15 mg/kg/h) for no more than 24 hours or 1-2 g IM every 3-12 hours (under supervision urine color: within 2 hours the urine becomes red; if no color change occurs, the injections are stopped). The concentration of iron in the patient's blood serum during this therapy usually decreases within 12–48 hours. The course and prognosis depend on the amount of the drug taken and the duration of its effect on the body.
ICD-10 • T45.4 Poisoning with iron and its compounds
How is iron deficiency treated?
It is necessary to identify and eliminate the cause of iron deficiency and adjust nutrition.
If these measures do not help, and iron loss is significant, your doctor may prescribe iron-based medications. Duration of treatment with iron preparations for the purpose of restoration and replenishment of iron reserves in the depot:
- for hidden iron deficiency 1-2 months,
- for mild anemia 3 months,
- with moderate anemia 4.5 months,
- for severe anemia 6 months.
Of course, first of all, iron supplements are used in the form of tablets, drops, capsules, syrups. If your doctor has prescribed you iron supplements, you need to be prepared for long-term and comfortable treatment. This will help by following simple rules.
The dosage of the drug is also determined by the presence of anemia (this is the full therapeutic dose) and the type of iron ion that is included in the drug.
What iron supplements are there?
Iron in preparations can be represented by a di- or trivalent ion, designated Fe2+ or Fe3+, which is part of salts or complex complexes (for example, polymaltose complex hydroxide).
When using iron salts, you may encounter problems with its absorption. Unfortunately, not all iron from the drug is absorbed in the gastrointestinal tract. Moreover, this process is poorly controlled, since it depends on the state of the gastrointestinal tract, food taken, and other medications. Therefore, both a lack of incoming iron and an overdose are possible. In addition, not all forms of medication are convenient to use; if some tablets are chewed, the enamel of the teeth is often stained and a metallic taste remains in the mouth for a long time. Trivalent iron preparations usually do not have these side effects.
A new generation of iron preparations – iron bisglycinate (chelate)
Anemia prevalence
The significance of anemia as a problem in the modern world is beyond doubt. Despite all the achievements of civilization, iron deficiency is the main and most common nutritional disorder in the world. Iron deficiency, which affects many children and women in developing countries, is the only type of nutritional deficiency that is also common in developed countries. Its prevalence levels are staggering: 2 billion people, more than 30% of the world's population, suffer from anemia.1
According to the World Health Organization, the most vulnerable groups for the development of anemia are pregnant women, women of reproductive age, children and the elderly. Moreover, the highest percentage of occurrence is among women of reproductive age - 30%.
Among anemias, the leading ones are iron deficiency, accounting for up to 90% in women and up to 80% among men. Important is the high prevalence of latent iron deficiency among the population, which ranges from 19.5% to 30%; in addition, from 50% to 86% of women have risk factors for anemia.
Iron deficiency anemia (IDA) is a disease of the blood system caused by iron deficiency in the body, accompanied by changes in the parameters of its metabolism, a decrease in the concentration of hemoglobin in erythrocytes, their quantitative and qualitative changes and is clinically expressed by anemic hypoxia and sideropenia.
Sideropenia and subsequent tissue and hemic hypoxia lead to disorders of the cardiovascular (myocardial dystrophy and circulatory disorders of varying degrees), nervous system (vegetative-vascular, vestibular disorders, asthenic syndrome), decreased reproductive function of women, as well as the development of complications during pregnancy and childbirth, changes in intelligence and behavioral moods, chronicity of various diseases and, as a consequence, decreased performance and deterioration in quality of life.4
The evolution of synthetic drugs for the treatment of iron deficiency anemia
Pharmacotherapy of IDA is based on the introduction of iron into the body from iron-containing drugs. The choice of a drug for the correction of sideropenia is given special importance, since not only effectiveness is important, but also the absence of adverse reactions and complications during their use.
There is a conditional division of iron preparations into divalent and trivalent. However, the valency of iron itself does not represent any value.
It is known that the absorption of iron in the intestine is possible only when the microelement is in a divalent form, which is able to pass through the cell membrane of the intestinal mucosa. The low pH value of gastric contents promotes the dissolution of alimentary iron and the transition of ferric iron (oxide) to the divalent form (ferrous).17
When gastric contents enter the intestine, the pH of the bolus increases and, unlike ferro-ion (Fe2+), ferri-ion (Fe3+) forms insoluble salts. Under these conditions, only mucin, by chelating iron, is able to maintain ferri ion in a soluble state.4
Thus, iron compounds in preparations must have good solubility, high bioavailability, sufficient elemental iron content and low toxicity. Let us consider the absorption features of each of the three known groups of iron preparations.
First generation of iron supplements
One of the first groups of iron preparations began to use ionic salts of divalent iron. This group is characterized by a fairly rapid onset of effect in terms of increasing hemoglobin and improving hemodynamic parameters in peripheral blood.
However, treatment with ionic iron preparations, in particular ferrous sulfate, causes adverse reactions in 44.7% of patients. The gastrointestinal tract (GIT) is most often affected. Symptoms of dysfunction of its upper sections usually appear within an hour after taking the medicine and can occur in either mild form (nausea, epigastric discomfort) or severe form - with abdominal pain and/or vomiting. In addition, ferrotherapy with iron salt preparations is often accompanied by the appearance of a metallic taste during the first days of treatment, darkening of tooth enamel and gums, and diarrhea or constipation are also possible. It is well known that iron salt preparations in the intestinal lumen interact with food components and medications, complicating the absorption of iron. In this regard, they are recommended to be prescribed 1 hour before meals, however, this increases the damaging effect of Fe2+ compounds on the intestinal mucosa, up to the development of its necrosis.5
The cause of these side effects is the hydrolysis of iron salts in the stomach. Under the influence of gastric juice, ionic iron salts undergo hydrolysis (dissociation) in the stomach, as a result of which free iron molecules negatively affect the gastrointestinal mucosa and provoke side effects: nausea, abdominal pain, metallic taste in the mouth, diarrhea/constipation.
Second generation of iron supplements
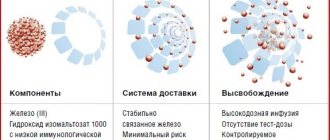
Absorption of iron as iron-III hydroxide-polymaltose complex (HPC)
has a fundamentally different scheme compared to its ionic compounds and is carried out through active absorption with competitive exchange of ligands, the level of which determines the rate of absorption of iron Fe3+.
The nonionic structure, which ensures the stability of the complex and the transfer of iron using a transport protein, prevents the free diffusion of iron ions in the body, that is, pro-oxidant reactions. However, the bioavailability of polymaltose iron-III complex is the lowest
among all iron preparations, only 10–15%.
Due to the large size of the molecule (55 kDa), its passive diffusion is approximately 40 times slower
than that of iron ions.6 Such low bioavailability must be compensated for by large daily doses of CHP.
New generation of iron supplements - a new solution to the problem of anemia
Since the late 90s and early 2000s, the use of chelated iron complexes began to be actively introduced for the treatment of iron deficiency and anemia in humans. Although this group of drugs appeared much earlier, and were initially used as food additives and in veterinary medicine.
In 1893, Alfred Werner postulated a new molecular structure to characterize these stable molecules. A few years later, in 1920, Morgan and Drew applied the term "chelate" to the molecular structure postulated by Werner. 7
Small protein molecules are easier to digest, so the body combines inorganic minerals with amino acids to take advantage of the intestines' affinity for protein absorption. This binding process is called chelation (key-lay-shun).10
Metal chelates are complex compounds of a metal with an amino acid.
Unlike metal salts, the ligand in the chelate complex donates electrons to the cation, thereby making the molecule ionic neutral, stable
to various factors acting in the gastrointestinal tract (pH, food), and the low molecular weight
promotes maximum absorption of iron
when taken orally.8
Chelated complexes penetrate more easily
through the intestinal wall and
are better absorbed
without disturbing the ionic and mineral balance of the cell.10
Ferrous bisglycinate consists of one molecule of iron, which is connected to the carboxyl groups of two molecules of glycine using covalent bonds.
The ratio of iron to ligand 1:2 neutralizes the valence of iron, which ensures its resistance to various factors acting in the gastrointestinal tract (pH, food). Therefore, the chelate compound cannot be hydrolyzed in the stomach, is completely absorbed in the small intestine and, unchanged, enters the enterocytes, where the iron molecule is released.8
Iron bisglycinate
is a source of non-heme iron. After oral administration, the compound enters unchanged into enterocytes, where it is hydrolyzed into iron and glycine. The stability of the iron bisglycinate compound is due to the fact that it does not hydrolyze at different pH values, and its low molecular weight (204 g/mol) allows for maximum absorption of iron when taken orally.8
- Thus, free iron molecules are not formed in the gastrointestinal tract.
- The absence of contact of non-ionized iron with the gastric mucosa minimizes possible side effects.
- Ferrous bisglycinate can bind to two types of receptors: DMT-1 (located on the duodenal villi) and PEPT-1 (localized throughout the gastrointestinal tract).9
- This feature of the chelate significantly increases the level of iron absorption in the gastrointestinal tract, which is 3.4 times higher than that of ferrous sulfate, which interacts only with DMT1 receptors.9
- The ionic neutral molecule does not react with other nutrients or interfere with their absorption.10
- Celiac disease provokes the occurrence of iron deficiency. Ferrous bisglycinate is the only drug that effectively corrects iron deficiency in patients with celiac disease through a dual absorption mechanism: binding to the DMT-1 and PEPT-1 receptors.11
- The bioavailability of iron bisglycinate approaches 90–100%. 9
Multizan® Ferrum contains iron bisglycinate, a patented Ferrochel® complex from Albion Minerals, a world leader and innovator in the field of mineral amino acid chelate nutrition.
Albion®'s unique range of chelated minerals:
- Maximum absorption and easy digestion.
- Does not interact with other nutrients.
- Resistant to the acidic environment of the stomach (pH).
- Suitable for vegetarians.
- Kosher certified product.
- Gluten free and GMO free.
- The safety of ferrous bisglycinate has been recognized by the EFSA (European Food Safety Authority) and the FDA (Food and Drug Administration, USA).12
Even with increased bioavailability, iron bisglycinate is safe. Absorption is controlled by iron stores
in the body, with larger amounts typically absorbed by people with lower iron status.
A body suffering from iron deficiency anemia may consume 90% of the iron, while a body without iron deficiency anemia may consume only 10%, or exactly as much as the body needs to compensate for metabolic losses. Ferrochel® iron bisglycinate has been found to be 2.6 times safer
than ferrous sulfate and safer than conventional inorganic iron found in foods and dietary supplements.13
Comparative table of LD50 doses (the average dose of a substance that causes the death of half of the members of the test group) of various iron preparations when administered orally to white mice.14, 15, 16
- Ferrous bisglycinate has the best safety profile
- Acute oral LD50 of Ferrochel iron bisglycinate 2800 mg/kg body weight - the highest dose of any iron supplement
- No Observed Side Effect Level (NOAEL) is at least 500 mg per kg body weight
Multizan® Ferrum - highly effective and easily absorbed iron with excellent tolerability
Literature:
- https://www. who. int/nutrition/topics/ida/ru/
- WHO global database of anemia, 1993-2005
- WHO meeting report. Preconception care to reduce maternal and childhood mortality and morbidity, 2012
- “Medicines used for the prevention and treatment of iron deficiency conditions” Kruglov D. S. Novosibirsk State Medical University. "Scientific review. Medical Sciences" No. 4/2017
- Gribakin S. G. The importance of baby food products fortified with iron in the prevention of iron deficiency anemia / S. G. Gribakin // Issues of modern pediatrics. — 2002. — vol. 1, No. 5. — pp. 52–56.
- https://medi. ru/info/3878/ Maltofer
- Stephen D. Ashmead The chemistry of ferrous bis-glycinate chelate Archivos Latinoamericanos de Nutrición ALAN v.51 n.1 supl.1 Caracas mar. 2001
- DeWayne HA Arch. Latino Am. De Nutr., 2001, 51 (1), 7-12
- Oscar Pineda, H. DeWayne Ashmead Effectiveness of Treatment of Iron-Deficiency Anemia in Infants and Young Children With Ferrous Bis-glycinate Chelate Nutrition 17:381–384, 2001
- https://www. albionferrochel. com/index. php/iron-importance/ferrochel
- M. Bertini, O. G. Shadrin “Correction of iron deficiency in children: expert opinion” 2018; https://health-ua.com/article/40109-korrektciya-defitcita-zheleza-u-detej-mnenie-ekspertov
- https://www.albionminerals. com/human-nutrition/products-trade/mineral-applications/dietary-supplements
- https://www.albionferrochel. com/index. php/effectiveness
- Geisser et al., 1992; Forster et al., 1993.
- Borzelleca, J, and Jeppsen, R., Food and Chemical Toxicology 37 (1999) 723-731 Safety evaluation of ferrous bisglycinate chelate
- Toxicology and safety of Ferrochel and other iron amino acid chelates Latinoarm. Nutr. 2001 Mar;51(1 Suppl 1):26-34.
- A. G. Rumyantseva, I. N. Zakharova “DIAGNOSTICS AND TREATMENT OF IRON DEFICIENCY ANEMIA IN CHILDREN AND ADOLESCENTS” Ministry of Health of the Russian Federation, study. manual, Moscow, 2015
return to news list
How to increase the effectiveness of treatment
To reduce the likelihood of side effects, iron salts are taken before meals.
The absorption of iron is higher when taken simultaneously with ascorbic and succinic acids, fructose, and cysteine. This property is used in some combination drugs.
Iron absorption is reduced by certain substances from food or medications. Thus, it is reduced by tannin (strongly brewed tea), calcium salts (in milk or medications), magnesium and manganese (in mineral complexes or antacid preparations, such as phosphalugel), tetracycline antibiotics, phosphoric and phytic acids (cereal seeds, legumes). This effect is smoothed out when using preparations based on ferric iron.
Iron absorption is increased in severe iron deficiency. Therefore, you should be more careful about the prescribed dosage. Doctors often rely on a therapeutic dose of 200 mg of iron ion/day. If you understand that an iron supplement is not suitable for you due to the development of adverse reactions, difficulties in taking it, or lack of improvement in your well-being, tell your doctor about this. Under no circumstances should you change the dosage of the drug yourself. Exceeding the dose can lead to overdose and poisoning, reducing the dose can lead to useless use and lack of effect.
How is the effectiveness of treatment assessed?
During the first days of treatment with iron supplements, physical sensations are assessed.
When treating anemia, on the 5-8th day of treatment, a reticulocyte crisis is determined (an increase in the number of reticulocytes compared to the initial value, usually by 2-3% or 20-30‰).
After 4 weeks of treatment, the increase in hemoglobin is assessed. An increase in hemoglobin concentration by 10 g/l by the end of the first month of treatment with iron preparations indicates that the dose has been correctly selected and the effectiveness of therapy. After hemoglobin is restored, therapy is continued to saturate the iron depot, while the dose of the drug is reduced by 2 times.
For hidden iron deficiency, half doses of the drugs are used for 4-8 weeks. Depot saturation is determined using a comprehensive biochemical study (ferritin, transferrin, TLC).
At Lab4U you can take tests to identify iron deficiency and monitor the effectiveness of therapy with a discount of up to 50%.
Introduction
Iron deficiency anemia (IDA) is one of the most common diseases in the world [1, 2], and among women of fertile age it is in first place in terms of incidence, often being a complication of pregnancy and childbirth, as well as a number of gynecological diseases [3–7] .
Another group of patients that especially often suffer from IDA are young children [8, 9], and in them IDA accounts for about 90% of all anemia. Nevertheless, any person, regardless of gender and age group, has a fairly high risk of encountering this problem at one stage or another in their life. Among the world's population, the incidence of IDA is unevenly distributed. According to experts from the World Health Organization (WHO), in developing countries it is several times higher than in developed countries. The range of fluctuations in the prevalence of IDA in the population ranges from 5 to 40% or more [10]. IDA is a polyetiological disease; it can have many different causes and predisposing factors [11]. These include socio-economic conditions, availability of medical care, nutritional habits, level of sanitary culture, prevalence of parasitic infestations and many others.
Etiology and pathogenesis of IDA
The development of IDA is associated with iron deficiency in the body, which in turn may be a consequence of impaired intake, absorption or increased loss of this microelement. In different sex and age groups, the predominant causes of IDA are distributed as follows:
- in women of fertile age [3–7]: heavy menstrual bleeding and menorrhagia (including due to pathologies of the reproductive system, such as endometriosis and fibroids),
- pregnancy (multiple pregnancies accompanied by early gestosis are in a special risk area),
- childbirth (especially repeated births with short intervals between them, as well as complicated by bleeding),
- miscarriages, frequent abortions,
- lactation;
- blood loss due to pathology of the gastrointestinal tract - gastrointestinal tract (esophageal bleeding, peptic ulcer of the stomach and duodenum, ulcerative colitis, Crohn's disease, polyposis, rectal fissures, hemorrhoids, tumors, etc.),
- iron deficiency at birth,
Iron plays an important role in the functioning of all organs and systems. It is a key element of the hemoglobin molecule, acting as a component of the protoporphyrin prosthetic group, responsible for the binding and transport of oxygen. Iron also takes part in the process of oxidative phosphorylation in cell mitochondria, in collagen synthesis, porphyrin metabolism, is present in significant quantities in muscle myoglobin, and is also necessary for the full functioning of the immune system [1]. And yet, the participation of iron in the processes of tissue respiration determines the main biological significance of this microelement. It is natural that with the development of IDA, one of the most significant problems becomes precisely tissue hypoxia and the pathology that it provokes [23–25].
A natural source of iron is food, which provides about 10–20 mg of this microelement daily in a normal, complete diet. Of this amount, no more than 10% (about 2 mg) is absorbed, which provides the daily need for iron. At the same time, most healthy menstruating women lose up to 20-30 mg of iron every month. A similar or even more critical situation may arise with some chronic diseases. It can be difficult to replace what is lost with iron from food, even if its content in the diet is quite high. The problem is aggravated in the presence of impaired absorption and absorption of iron. A gradually developing imbalance between the intake and loss of iron depletes its reserves in the body, leading to an iron deficiency state, in particular to IDA with progressive hemic hypoxia and accompanying metabolic disorders.
Depending on the predominant mechanism of development of iron deficiency, anemia is divided into the following groups [25]:
- associated with blood loss;
- associated with impaired iron absorption;
- associated with an increased need for iron;
- nutritionally dependent.
In some cases, signs from several groups with varying degrees of severity may occur.
Clinical manifestations and diagnosis of IDA
The main clinical manifestations of IDA are characterized by hypoxic and sideropenic syndromes [1, 2, 27, 28].
Signs that make up hypoxic syndrome:
- pallor of the skin and visible mucous membranes;
- cardiopalmus;
- noise in ears;
- headache, dizziness;
- general weakness;
- loss of appetite;
- decreased performance, concentration, memory impairment.
Components of sideropenic syndrome:
- perversion of taste and smell;
- dry skin, pigmentation (“coffee with milk”);
- deformations and changes in the structure of nails (cross-striations, concavity, thinning, fragility);
- deterioration of hair condition (fragility, dullness, split ends, hair loss up to alopecia);
- manifestations of angular stomatitis (“jams”);
- burning sensation in the tongue.
In addition to the listed symptoms, the development of neurosis-like disorders, the manifestation of neurasthenia, disorders of peripheral circulation and microcirculation, decreased tolerance to physical activity, changes in metabolism in the myocardium are also possible, leading with long-term IDA to increasing symptoms of myocardial dystrophy, as well as shifts in the autonomic regulation of cardiac activity towards sympathicotonia .
Negative changes in the functioning of the gastrointestinal tract are noted. Patients with IDA often suffer from chronic gastritis and intestinal pathology. Syndromes of impaired absorption of iron in the intestine are often secondary in nature, further aggravating iron deficiency [17]. Regenerative disorders in the gastric mucosa are currently considered as the causes of decreased secretion and acid formation in IDA.
IDA negatively affects the immune system [29]. Thus, iron deficiency indirectly disrupts the functioning of the cellular component of anti-infective immunity: the proliferation of lymphocytes slows down and the microbicidal activity of granulocytes decreases. This may lead to a slight increase in the risk of developing infections and their unfavorable course.
The listed variety of clinical manifestations of IDA, which sharply reduce the quality of life of patients, once again emphasizes the particular importance of timely diagnosis and full correction of this condition. A separate problem is the low specificity of most clinical symptoms [28], which makes it difficult to confidently diagnose IDA solely on their basis.
In this regard, laboratory data, incl. general blood analysis:
- assessment of hematocrit (diagnostic sign - its decrease);
- study of the number of red blood cells and reticulocytes in the blood (the number of red blood cells may be within normal limits or reduced, reticulocytosis is not typical, but can be observed with bleeding);
- determination of the average concentration and average content of hemoglobin in erythrocytes (these indicators are reduced, which reflects hypochromia of erythrocytes); determination of the shape and size of red blood cells (characteristic of anisocytosis with a tendency to microcytosis).
If IDA is suspected, it is also recommended to examine such indicators of iron metabolism as ferritin level (decreases), serum transferrin and serum iron-binding capacity (increases), serum iron and transferrin iron saturation coefficient (decreases).
For successful treatment, it is also important to determine the cause of the development of IDA, possible sources of blood loss, and concomitant diseases. For this purpose, X-ray examination of the chest organs, ultrasound examination of the abdominal organs, pelvis, and retroperitoneal space are used; electrocardiography, esophagogastroduodenoscopy, colonoscopy, as well as biochemical blood test, general urine test, etc. The results of basic studies help to choose the direction of further diagnostic search.
Treatment of IDA
The goal of treatment is to introduce iron into the body in an amount sufficient to normalize hemoglobin levels and replenish tissue iron stores [30–32]. At the same time, normal hemoglobin levels for women are 120–140 g/l, for men – 130–160 g/l, and a serum ferritin level above 40–60 μg/l indicates satisfactory accumulation of iron in the depot. This review examines the correction of IDA exclusively by pharmacological means. However, in some cases (severe IDA, concomitant cardiovascular pathology with the threat of decompensation due to iron deficiency, etc.), concomitant therapy with erythropoietin drugs, as well as blood transfusion therapy, can be prescribed on an individual basis.
When starting treatment for IDA, it is necessary to remember that anemia cannot be corrected with vitamin and mineral complexes containing iron. The vast majority of patients with IDA require special iron-containing medications [32]. Oral administration is a priority as a more convenient and safe way to use such drugs [33–35]. Parenteral iron preparations are used only if there are contraindications to oral administration, their poor tolerability or ineffectiveness. In this case, only the intravenous route of administration is recommended. Intramuscular injections of iron-containing drugs are ineffective and dangerous, because can cause infiltrates and even the development of myosarcomas.
Currently, preference is given to therapeutic regimens that involve taking 100–200 mg of elemental iron per day for adults (the optimal dose recommended by WHO is 120 mg/day). The use of more than 300 mg of iron per day is not advisable, because this usually does not increase the volume of absorption, while at the same time increasing the risk of developing undesirable side effects (stool disorders, signs of gastric irritation, etc.) [36]. When calculating the daily and course doses, the severity of the anemic syndrome, visceral lesions, and serum iron levels are taken into account.
The modern pharmaceutical industry offers a fairly wide range of iron preparations, which differ in their quantitative (high- and low-dose) and qualitative compositions (single-component, combined). Oral iron preparations are also conventionally divided into ionic (salt) divalent (of iron salts, sulfate is most often used) and non-ionic (non-salt) ferric iron preparations based on polymaltose complex hydroxide (HPC) and protein succinylate [37, 38]. Randomized studies in recent years have proven equal effectiveness of both groups. For a more successful result, it is advisable to prescribe vitamins in parallel with iron supplements. For example, ascorbic acid allows for better absorption and assimilation of iron [39] and the full course of plastic processes in the body.
It should be remembered that the therapeutic effect of iron supplements, as a rule, develops quite slowly, so ferrotherapy is a long process. Depending on the severity of iron deficiency, treatment can last from 1 to 3 months or more. At the same time, data are accumulating on the successful use of low-dose drugs in intermittent courses (2 weeks per month), as well as on the benefits of treatment in an alternating mode - every other day for a month [40]. The listed regimens provide a much lower frequency of side effects without loss of effectiveness. To avoid side effects and to increase patient adherence to therapy, it makes sense to refrain from unnecessarily prescribing high-dose drugs and multiple doses throughout the day.
WHO experts recommend the use of drugs with sustained release of iron [41]. This allows you to reduce the frequency of taking the drug, while simultaneously improving its absorption and tolerability. The duration of their use is determined individually, taking into account laboratory parameters. The main course of treatment continues until the optimal hemoglobin level is achieved and iron metabolism in the blood serum is restored. Further, in order to replenish tissue depots, it may be necessary to extend the drug intake for up to 2 months. If iron deficiency is severe, the total duration of treatment with oral sustained-release iron medications is usually 3 to 6 months [37, 41, 42].
Monitoring the effectiveness of treatment for IDA
Monitoring the effectiveness of treatment with iron supplements is carried out by monitoring the following laboratory parameters [42]:
- hemogram;
- ferritin;
- iron binding capacity of serum;
- transferrin.
With adequate therapy, patients’ well-being improves already on the 5th–6th day of taking the drug. On the 7th–12th day, one should expect a reticulocyte crisis - an increase in reticulocytes by 2 or more times from the initial values. Hemoglobin concentration begins to increase after approximately 2.5–3 weeks of treatment. By the end of the 4th week it should increase by 10 g/l. Complete normalization of this indicator is usually observed no earlier than a month from the start of treatment. Hemogram values in mild anemia usually return to normal after 5–8 weeks of ferrotherapy. It is important to dynamically monitor hemoglobin levels during treatment and after completion of the course. This indicator should be monitored monthly throughout the year, which makes it possible to decide on the need for maintenance treatment.
If the target hemoglobin level and normal serum ferritin level cannot be achieved, conditions that interfere with iron absorption, as well as the possibility of adverse drug interactions (for example, with antacids), should be excluded.
Prevention of IDA
Primary prevention of IDA and latent iron deficiency is, first of all, a complete, balanced diet for a person at any age. Secondary prevention of IDA is facilitated by regular preventive examinations of the population for the purpose of timely diagnosis and treatment of diseases that can lead to iron deficiency in the body. In this case, screening studies to identify iron deficiency conditions occupy a central place [43]. You should remember about risk groups for the development of latent iron deficiency and IDA. These include people with irreparable causes of iron deficiency (chronic blood loss, malabsorption syndrome, celiac disease, program hemodialysis, inoperable tumors, etc.), vegans and vegetarians, regular blood donors, and pregnant women. They are recommended to take additional preventive iron supplements.
Conclusion
IDA is a very serious and quite common problem, and the medical community is currently actively searching for optimal ways to solve it. Some progress has already been made in the development of effective and safe drugs for the treatment and prevention of iron deficiency conditions. Competent pharmacological correction of IDA can improve the quality of life and increase performance in the vast majority of patients with a similar diagnosis. In this case, it is advisable to use modern drugs, the production technologies of which ensure the maximum possible bioavailability of iron, and also minimize the risk of developing the most common side effects from ferrotherapy.